Abstract
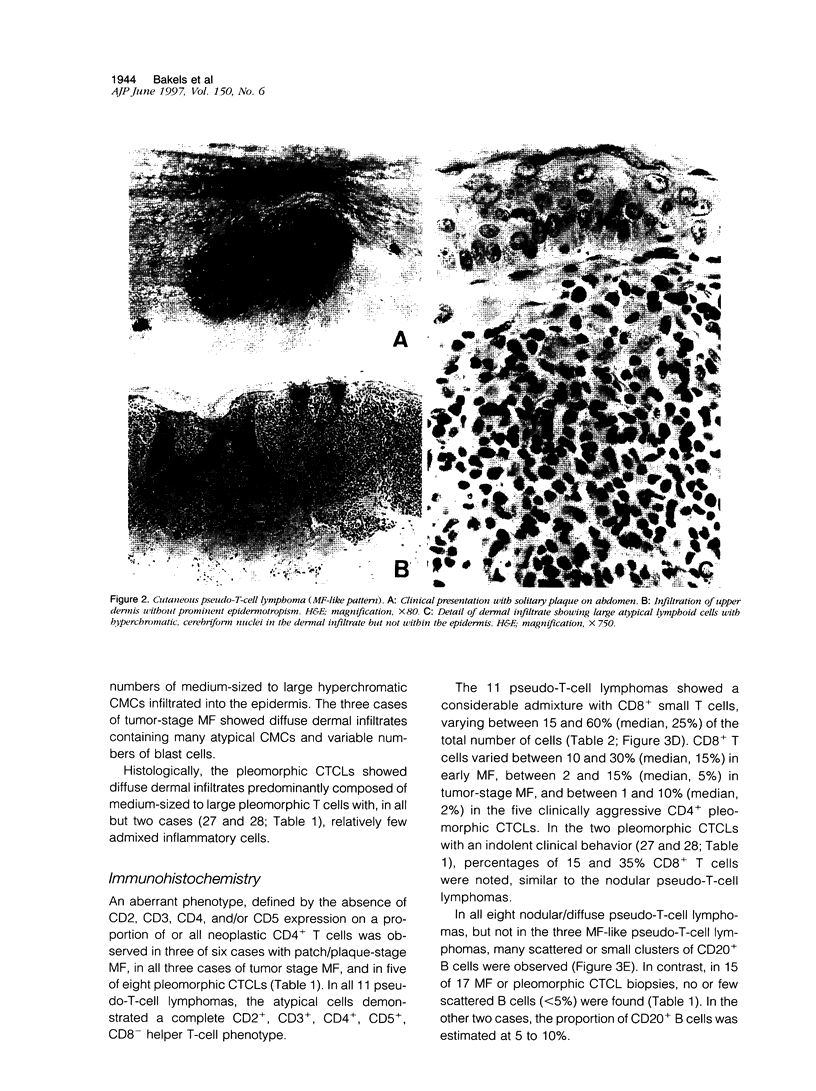
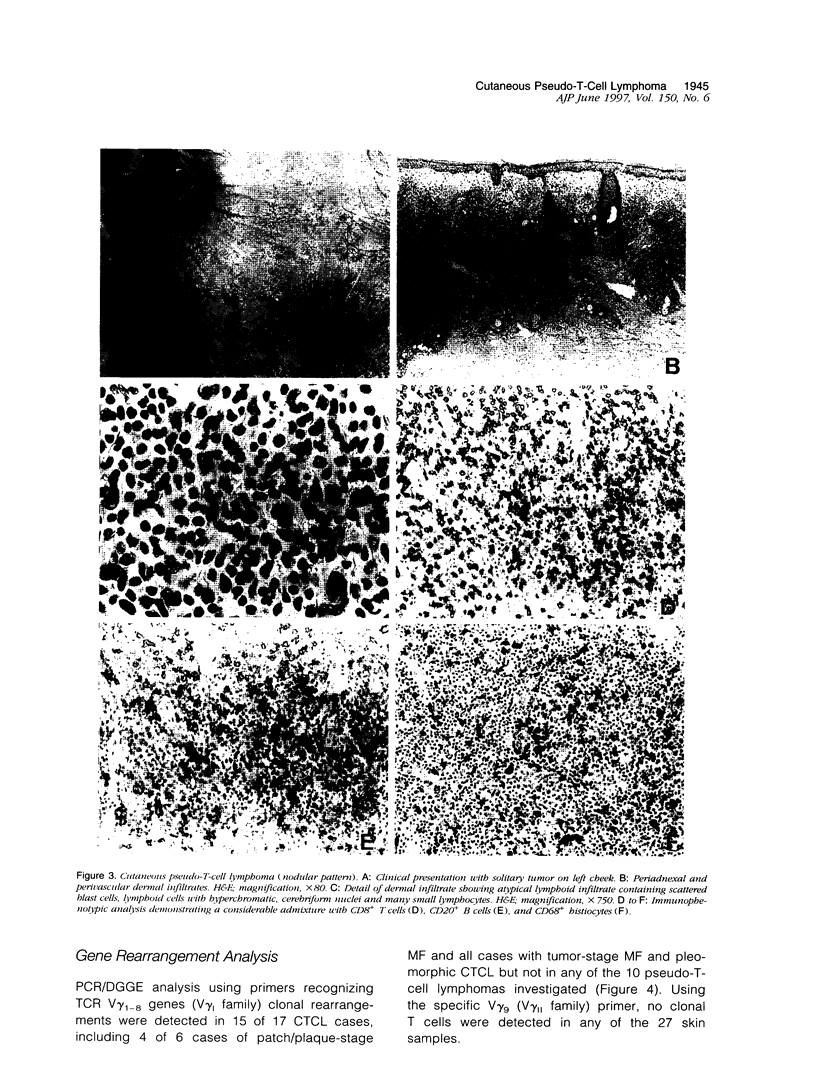
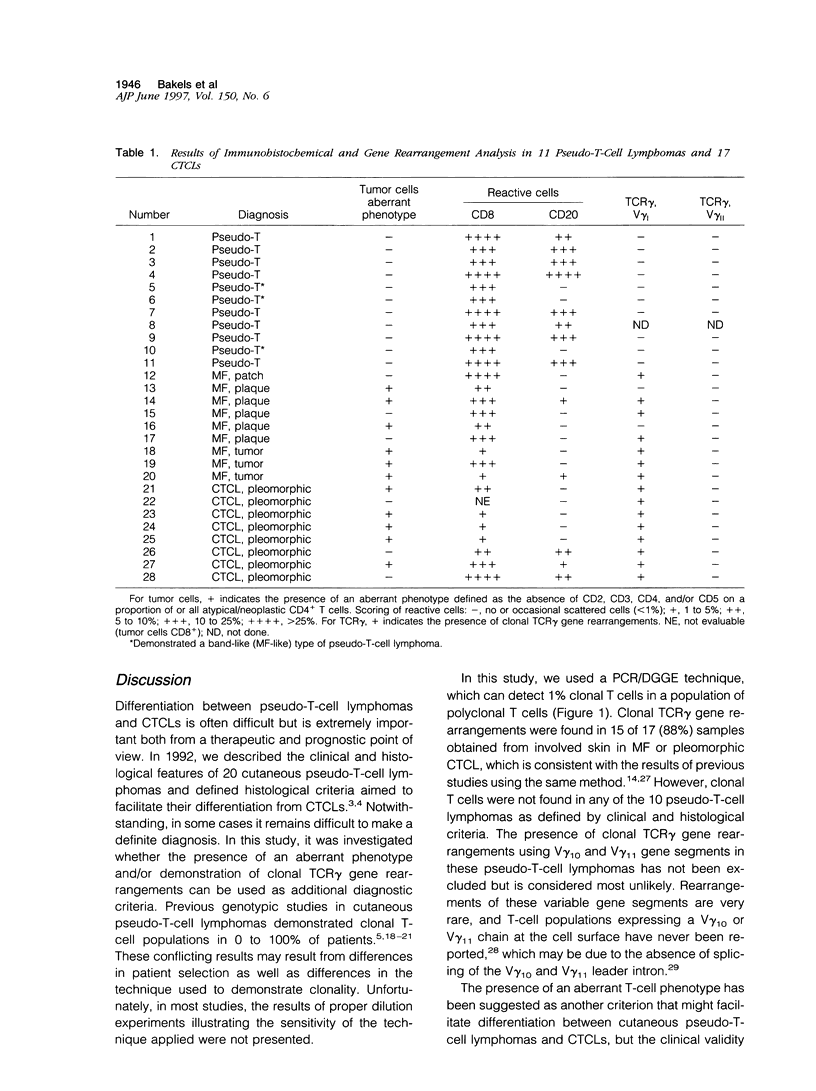
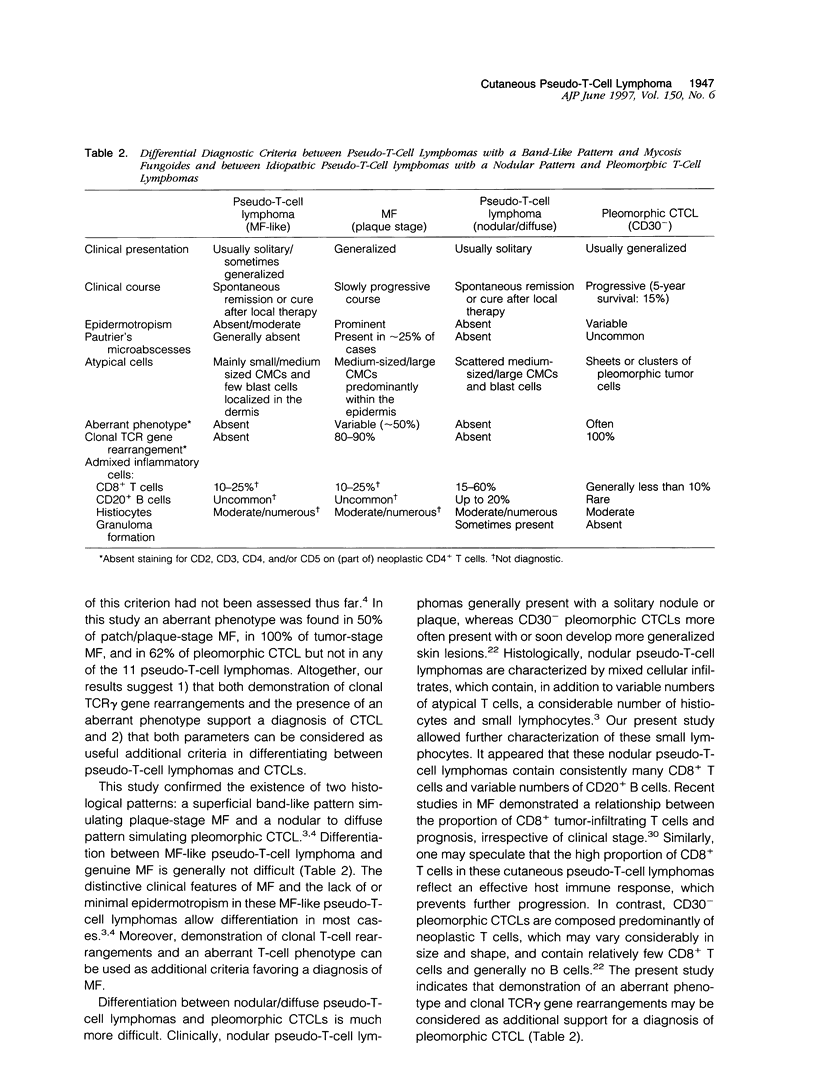
Differentiation between cutaneous pseudo-T-cell lymphomas and cutaneous T-cell lymphomas (CTCLs) may be extremely difficult. In this study, it was investigated whether demonstration of an aberrant phenotype and detection of clonal T-cell receptor gamma (TCR gamma) gene rearrangements can be used as additional differential diagnostic criteria. Immunohistochemical studies and TCR gamma gene rearrangement analysis using a polymerase chain reaction with primers specific for V gamma 1-8 and V gamma 9 gene segments in combination with denaturing gradient gel electrophoresis (PCR/ DGGE) were performed on frozen material of 11 pseudo-T-cell lymphomas and 17 CTCLs, including 9 cases of mycosis fungoides (MF) and 8 pleomorphic CTCLs. Clonal TCR gamma gene rearrangements were found in 66% of patch/plaque-stage MF and 100% of tumor-stage MF and pleomorphic CTCL, but not in any of 10 pseudo-T-cell lymphomas studied. Aberrant expression of CD2, CD3, and/or CD5 antigens was noted in 3 of 6 (50%) cases of patch/plaque-stage MF, all three cases of tumor-stage MF, and 5 of 8 (62%) pleomorphic CTCLs, but not in any of the 11 pseudo-T-cell lymphomas. Moreover, in pseudo-T-cell lymphomas exhibiting a nodular or diffuse growth pattern, a considerable admixture with reactive CD8+ T cells (15 to 60%), B cells (up to 20%), and macrophages was a characteristic finding. In conclusion, the results of this study suggest that demonstration of clonal TCR gene rearrangements and an aberrant phenotype, as well as demonstration of many admixed CD8+ T cells and B cells can be considered as useful additional criteria in the differentiation between pseudo-T-cell lymphomas and CTCLs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman A. B., Breza T. S., Capland L. Spongiotic simulants of mycosis fungoides. Arch Dermatol. 1974 Feb;109(2):218–220. [PubMed] [Google Scholar]
- Bakels V., Van Oostveen J. W., Geerts M. L., Gordijn R. L., Walboomers J. M., Scheffer E., Meijer C. J., Willemze R. Diagnostic and prognostic significance of clonal T-cell receptor beta gene rearrangements in lymph nodes of patients with mycosis fungoides. J Pathol. 1993 Jul;170(3):249–255. doi: 10.1002/path.1711700306. [DOI] [PubMed] [Google Scholar]
- Bakels V., van Oostveen J. W., Gordijn R. L., Walboomers J. M., Meijer C. J., Willemze R. Diagnostic value of T-cell receptor beta gene rearrangement analysis on peripheral blood lymphocytes of patients with erythroderma. J Invest Dermatol. 1991 Nov;97(5):782–786. doi: 10.1111/1523-1747.ep12486767. [DOI] [PubMed] [Google Scholar]
- Beljaards R. C., Meijer C. J., Van der Putte S. C., Hollema H., Geerts M. L., Bezemer P. D., Willemze R. Primary cutaneous T-cell lymphoma: clinicopathological features and prognostic parameters of 35 cases other than mycosis fungoides and CD30-positive large cell lymphoma. J Pathol. 1994 Jan;172(1):53–60. doi: 10.1002/path.1711720110. [DOI] [PubMed] [Google Scholar]
- Bignon Y. J., Souteyrand P. Genotyping of cutaneous T-cell lymphomas and pseudolymphomas. Curr Probl Dermatol. 1990;19:114–123. doi: 10.1159/000418081. [DOI] [PubMed] [Google Scholar]
- Bourguin A., Tung R., Galili N., Sklar J. Rapid, nonradioactive detection of clonal T-cell receptor gene rearrangements in lymphoid neoplasms. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8536–8540. doi: 10.1073/pnas.87.21.8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incan M., Souteyrand P., Bignon Y. J., Fonck Y., Roger H. Hydantoin-induced cutaneous pseudolymphoma with clinical, pathologic, and immunologic aspects of Sézary syndrome. Arch Dermatol. 1992 Oct;128(10):1371–1374. [PubMed] [Google Scholar]
- Dommann S. N., Dommann-Scherrer C. C., Dours-Zimmermann M. T., Zimmermann D. R., Kural-Serbes B., Burg G. Clonal disease in extracutaneous compartments in cutaneous T-cell lymphomas. A comparative study between cutaneous T-cell lymphomas and pseudo lymphomas. Arch Dermatol Res. 1996 Apr;288(4):163–167. doi: 10.1007/BF02505218. [DOI] [PubMed] [Google Scholar]
- Fisher A. A. Allergic contact dermatitis mimicking mycosis fungoides. Cutis. 1987 Jul;40(1):19–21. [PubMed] [Google Scholar]
- Griesser H., Feller A. C., Sterry W. T-cell receptor and immunoglobulin gene rearrangements in cutaneous T-cell-rich pseudolymphomas. J Invest Dermatol. 1990 Sep;95(3):292–295. doi: 10.1111/1523-1747.ep12484964. [DOI] [PubMed] [Google Scholar]
- Hoppe R. T., Medeiros L. J., Warnke R. A., Wood G. S. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol. 1995 Mar;32(3):448–453. doi: 10.1016/0190-9622(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Huck S., Dariavach P., Lefranc M. P. Variable region genes in the human T-cell rearranging gamma (TRG) locus: V-J junction and homology with the mouse genes. EMBO J. 1988 Mar;7(3):719–726. doi: 10.1002/j.1460-2075.1988.tb02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerl H., Smolle J. Classification of cutaneous pseudolymphomas. Curr Probl Dermatol. 1990;19:167–175. doi: 10.1159/000418088. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Orbaneja J. G., Diez L. I., Lozano J. L., Salazar L. C. Lymphomatoid contact dermatitis: a syndrome produced by epicutaneous hypersensitivity with clinical features and a histopathologic picture similar to that of mycosis fungoides. Contact Dermatitis. 1976 Jun;2(3):139–143. doi: 10.1111/j.1600-0536.1976.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J. U., Scheffer E., Meijer C. J., Willemze R. Cutaneous pseudo-T-cell lymphomas. A clinicopathologic study of 20 patients. Cancer. 1992 Feb 1;69(3):717–724. doi: 10.1002/1097-0142(19920201)69:3<717::aid-cncr2820690319>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J. U., Willemze R. Cutaneous pseudolymphomas: classification and differential diagnosis. Semin Dermatol. 1994 Sep;13(3):187–196. [PubMed] [Google Scholar]
- Rijlaarsdam U., Scheffer E., Meijer C. J., Kruyswijk M. R., Willemze R. Mycosis fungoides-like lesions associated with phenytoin and carbamazepine therapy. J Am Acad Dermatol. 1991 Feb;24(2 Pt 1):216–220. doi: 10.1016/0190-9622(91)70029-2. [DOI] [PubMed] [Google Scholar]
- Smolle J., Torne R., Soyer H. P., Kerl H. Immunohistochemical classification of cutaneous pseudolymphomas: delineation of distinct patterns. J Cutan Pathol. 1990 Jun;17(3):149–159. doi: 10.1111/j.1600-0560.1990.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Sterry W., Staib G. Moderne molekular-biologische Diagnostik kutaner maligner Lymphome. Hautarzt. 1995 Jan;46(1):4–9. doi: 10.1007/s001050050199. [DOI] [PubMed] [Google Scholar]
- Theodorou I., Delfau-Larue M. H., Bigorgne C., Lahet C., Cochet G., Bagot M., Wechsler J., Farcet J. P. Cutaneous T-cell infiltrates: analysis of T-cell receptor gamma gene rearrangement by polymerase chain reaction and denaturing gradient gel electrophoresis. Blood. 1995 Jul 1;86(1):305–310. [PubMed] [Google Scholar]
- Toonstra J., Henquet C. J., van Weelden H., van der Putte S. C., van Vloten W. A. Actinic reticuloid. A clinical photobiologic, histopathologic, and follow-u study of 16 patients. J Am Acad Dermatol. 1989 Aug;21(2 Pt 1):205–214. [PubMed] [Google Scholar]
- Toonstra J., van Weelden H., Gmelig Meyling F. H., van der Putte S. C., Schiere S. I., Baart de la Faille H. Actinic reticuloid simulating Sézary syndrome. Report of two cases. Arch Dermatol Res. 1985;277(3):159–166. doi: 10.1007/BF00404310. [DOI] [PubMed] [Google Scholar]
- Willemze R., Beljaards R. C., Meijer C. J. Classification of primary cutaneous T-cell lymphomas. Histopathology. 1994 May;24(5):405–415. doi: 10.1111/j.1365-2559.1994.tb00549.x. [DOI] [PubMed] [Google Scholar]
- Wolff-Sneedorff A., Ralfkiaer E., Thomsen K., Vejlsgaard G. L. Analyses of T-cell receptor beta-chain genes by Southern blotting in known and suspected cutaneous T-cell lymphoma. A study of 67 samples from 32 patients. Clin Exp Dermatol. 1995 Mar;20(2):115–122. doi: 10.1111/j.1365-2230.1995.tb02667.x. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Tung R. M., Haeffner A. C., Crooks C. F., Liao S., Orozco R., Veelken H., Kadin M. E., Koh H., Heald P. Detection of clonal T-cell receptor gamma gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J Invest Dermatol. 1994 Jul;103(1):34–41. doi: 10.1111/1523-1747.ep12389114. [DOI] [PubMed] [Google Scholar]
- Zhang X. M., Tonnelle C., Lefranc M. P., Huck S. T cell receptor gamma cDNA in human fetal liver and thymus: variable regions of gamma chains are restricted to V gamma I or V9, due to the absence of splicing of the V10 and V11 leader intron. Eur J Immunol. 1994 Mar;24(3):571–578. doi: 10.1002/eji.1830240312. [DOI] [PubMed] [Google Scholar]
- van der Putte S. C., Toonstra J., Felten P. C., van Vloten W. A. Solitary nonepidermotropic T cell pseudolymphoma of the skin. J Am Acad Dermatol. 1986 Mar;14(3):444–453. doi: 10.1016/s0190-9622(86)70055-8. [DOI] [PubMed] [Google Scholar]
- van der Putte S. C., Toonstra J., van Wichen D. F., van Unnik J. A., van Vloten W. A. Aberrant immunophenotypes in mycosis fungoides. Arch Dermatol. 1988 Mar;124(3):373–380. doi: 10.1001/archderm.124.3.373. [DOI] [PubMed] [Google Scholar]