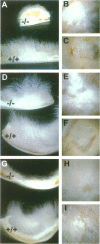
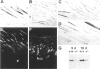
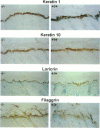
Abstract
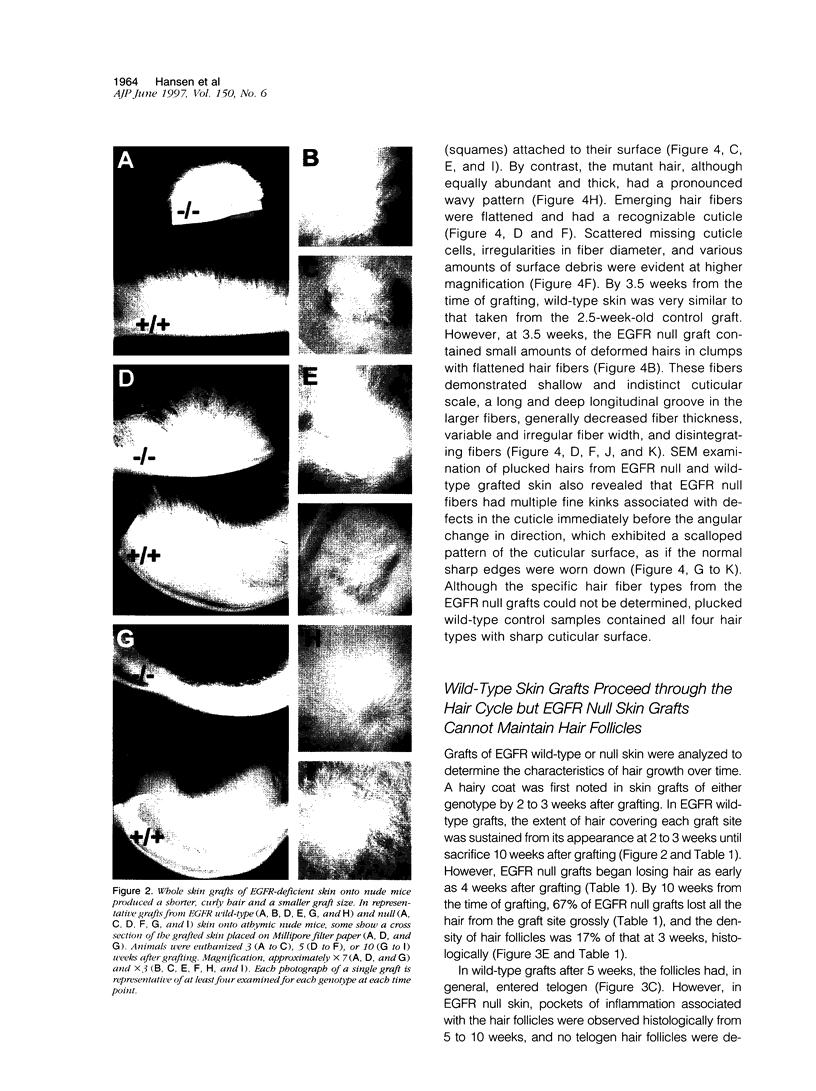
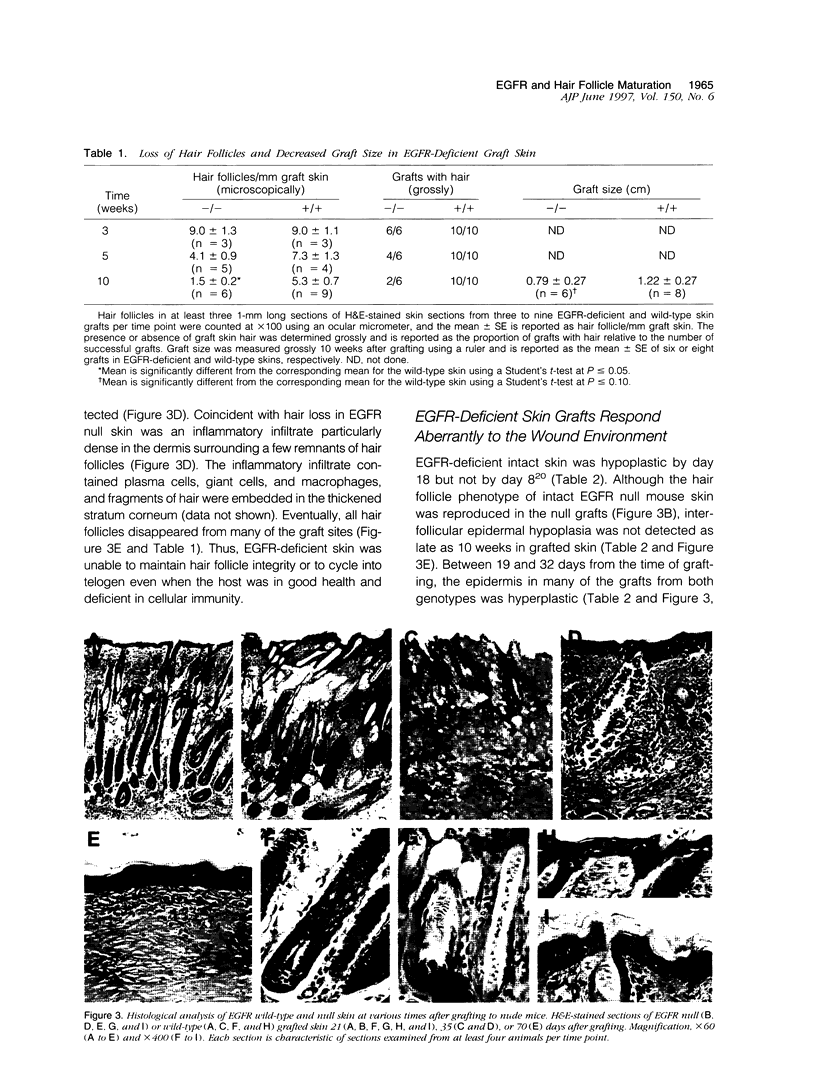
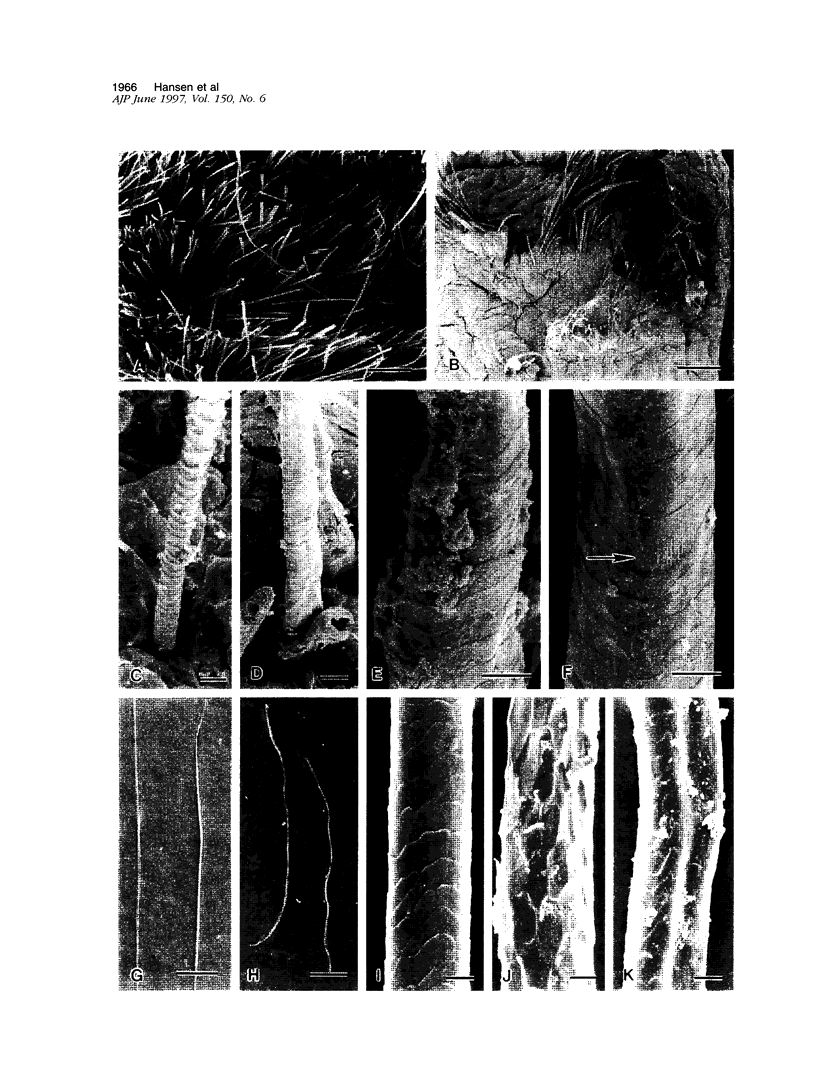
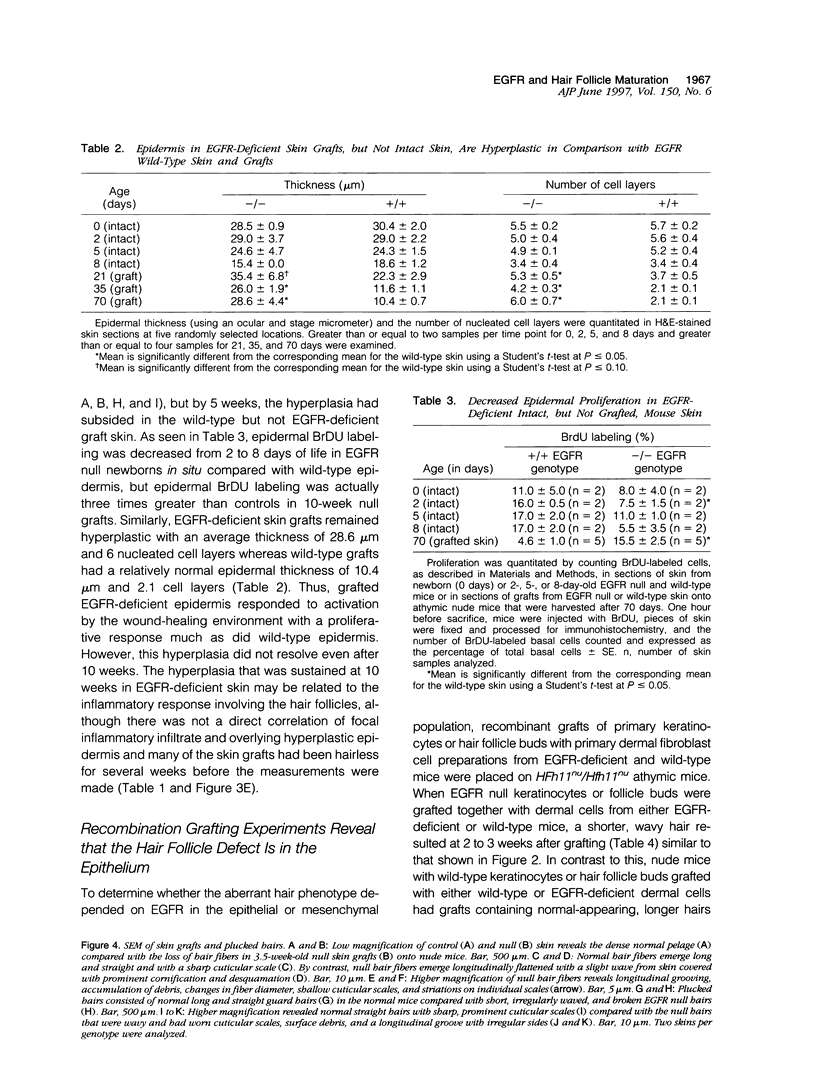
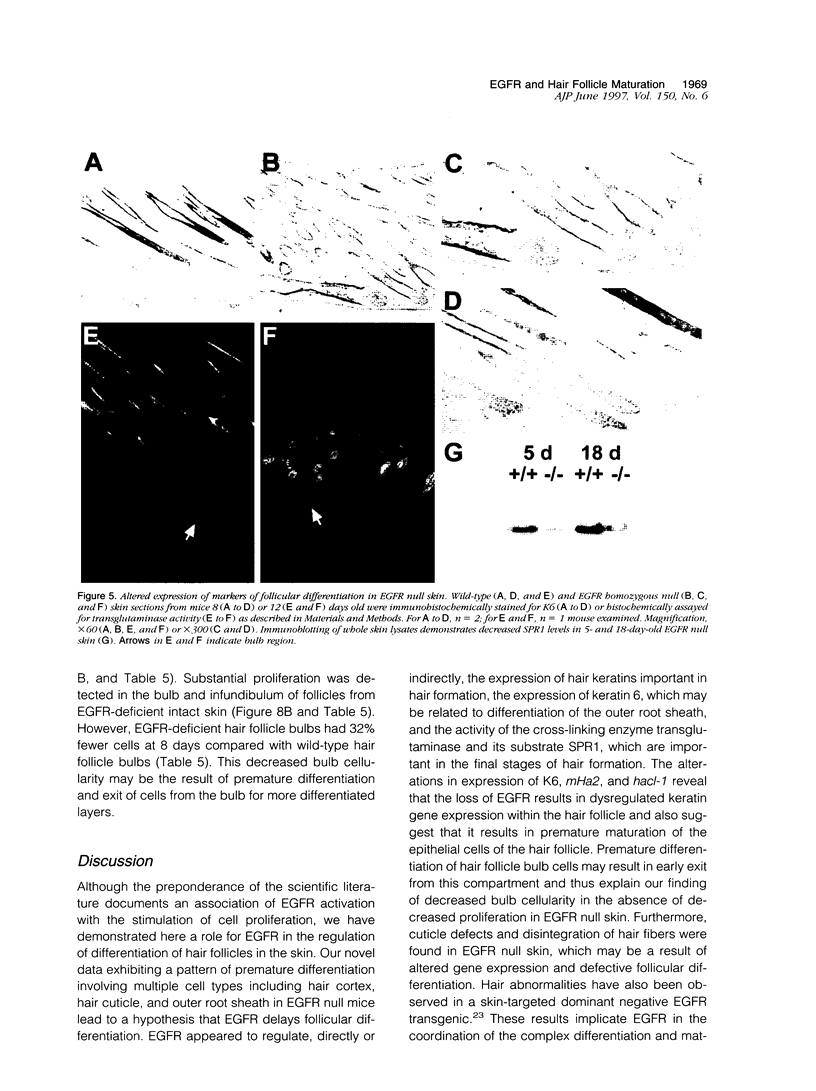
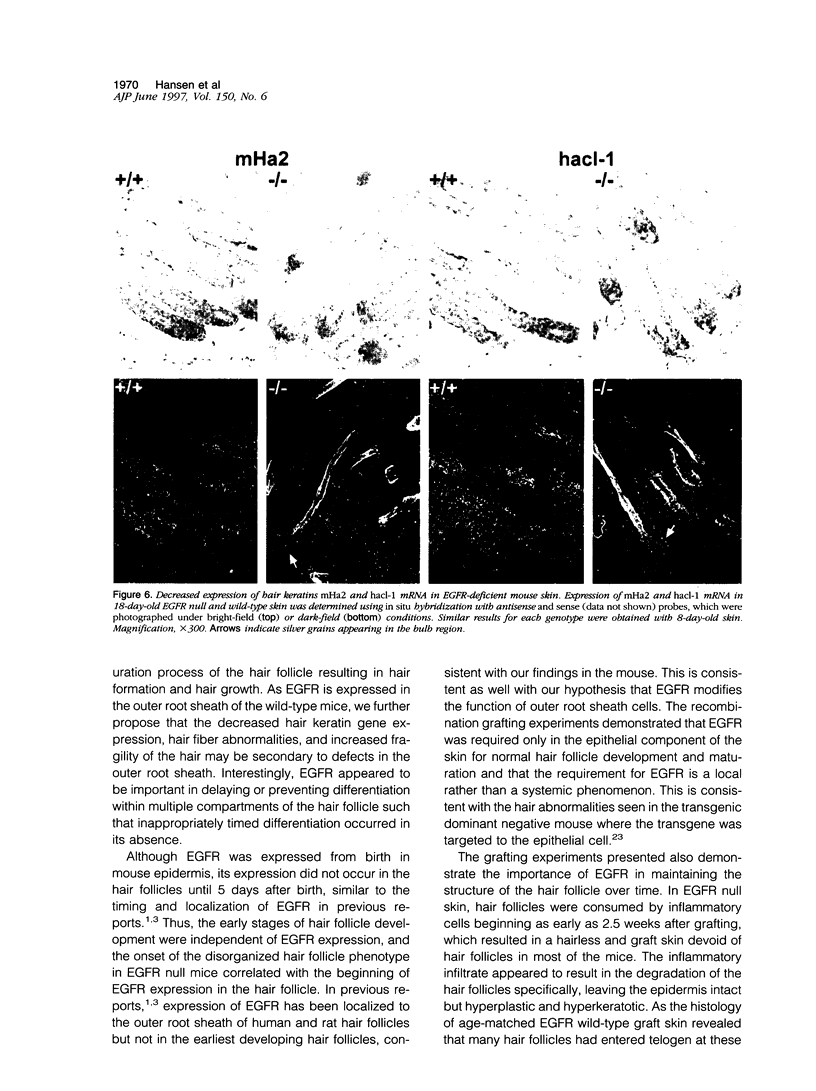
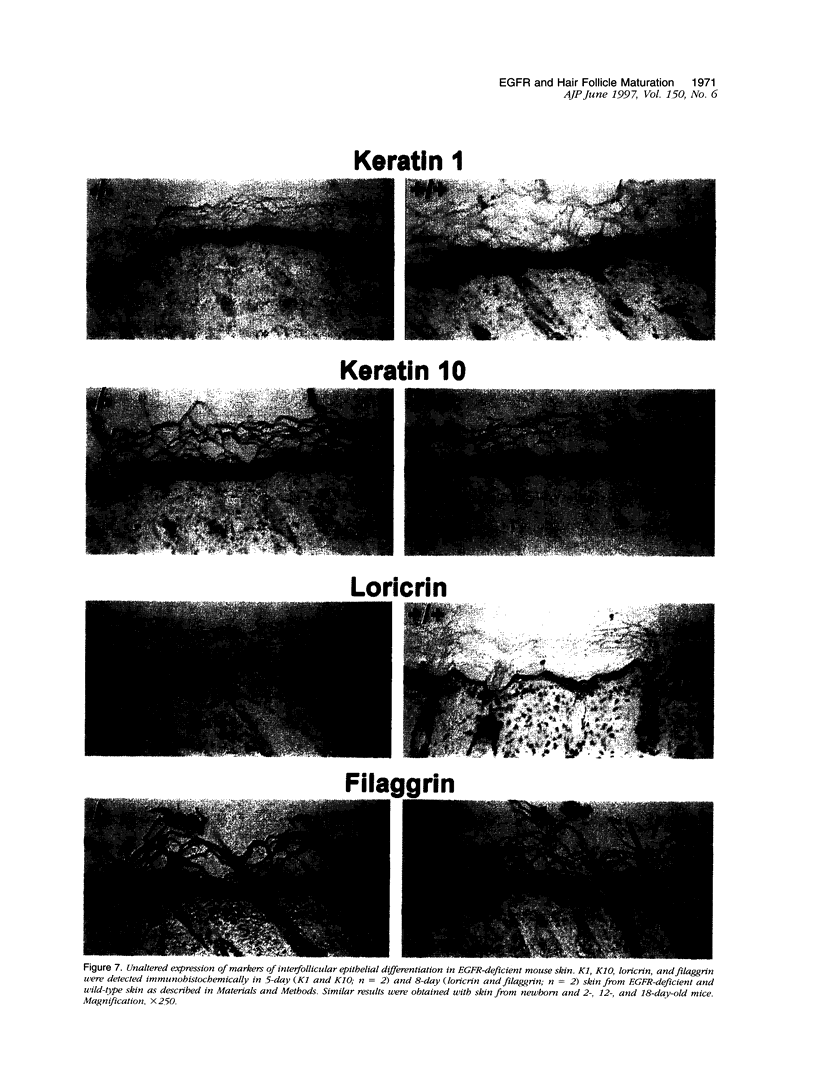
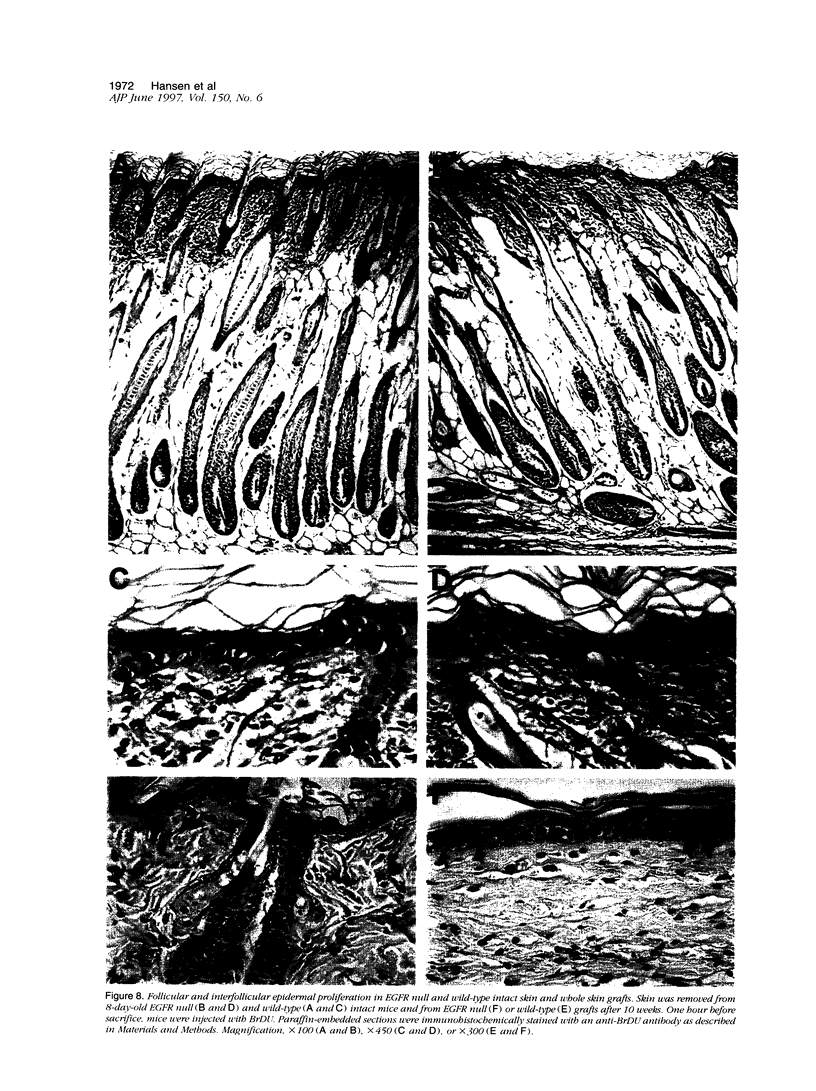
Mice harboring a targeted disruption of the epidermal growth factor receptor (EGFR) allele exhibit a severely disorganized hair follicle phenotype, fuzzy coat, and systemic disease resulting in death before 3 weeks. This skin phenotype was reproduced in whole skin grafts and in grafts of EGFR null hair follicle buds onto nude mice, providing a model to evaluate the natural evolution of skin lacking the EGFR. Hair follicles in grafts of null skin did not progress from anagen to telogen and scanning electron micrografts revealed wavy, flattened hair fibers with cuticular abnormalities. Many of the EGFR null hair follicles in the grafted skin were consumed by an inflammatory reaction resulting in complete hair loss in 67% of the grafts by 10 weeks. Localization of follicular differentiation markers including keratin 6, transglutaminase, and the hair keratins mHa2 and hacl-1 revealed a pattern of premature differentiation within the null hair follicles. In intact EGFR null mice, proliferation in the interfollicular epidermis, but not hair follicles, was greatly decreased in the absence of EGFR. In contrast, grafting of EGFR null skin resulted in a hyperplastic response in the epidermis that did not resolve even after 10 weeks, although the wound-induced hyperplasia in EGFR wild-type grafts had resolved within 3 to 4 weeks. Thus, epithelial expression of the EGFR has complex functions in the skin. It is important in delaying follicular differentiation, may serve to protect the hair follicle from immunological reactions, and modifies both normal and wound-induced epidermal proliferation but seems dispensable for follicular proliferation.
Full text
PDF



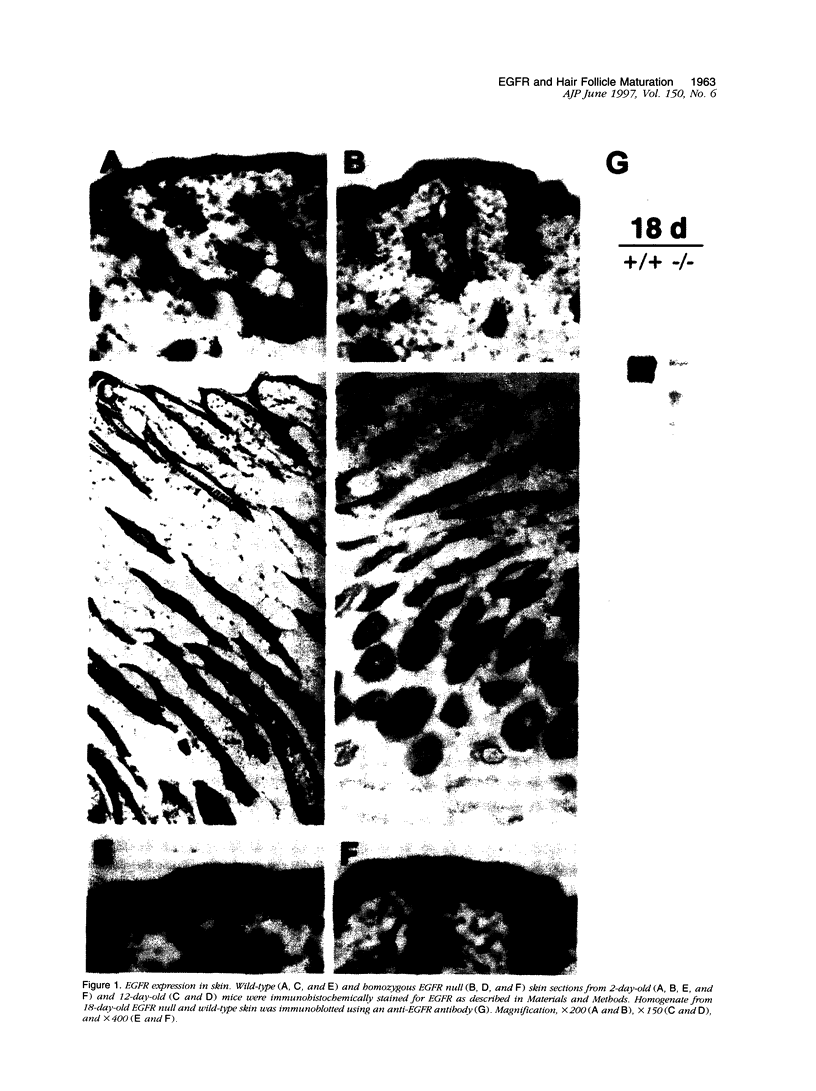



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Bernard B. A., Reano A., Darmon Y. M., Thivolet J. Precocious appearance of involucrin and epidermal transglutaminase during differentiation of psoriatic skin. Br J Dermatol. 1986 Mar;114(3):279–283. doi: 10.1111/j.1365-2133.1986.tb02818.x. [DOI] [PubMed] [Google Scholar]
- Carroll J. M., Romero M. R., Watt F. M. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995 Dec 15;83(6):957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- Chen J. D., Kim J. P., Zhang K., Sarret Y., Wynn K. C., Kramer R. H., Woodley D. T. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993 Dec;209(2):216–223. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol. 1965 Dec;12(3):394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- Cook P. W., Pittelkow M. R., Shipley G. D. Growth factor-independent proliferation of normal human neonatal keratinocytes: production of autocrine- and paracrine-acting mitogenic factors. J Cell Physiol. 1991 Feb;146(2):277–289. doi: 10.1002/jcp.1041460213. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., McCarthy K. J., Woods A. Proteoglycans and glycoproteins in hair follicle development and cycling. Ann N Y Acad Sci. 1991 Dec 26;642:243–252. doi: 10.1111/j.1749-6632.1991.tb24391.x. [DOI] [PubMed] [Google Scholar]
- Dlugosz A. A., Cheng C., Denning M. F., Dempsey P. J., Coffey R. J., Jr, Yuspa S. H. Keratinocyte growth factor receptor ligands induce transforming growth factor alpha expression and activate the epidermal growth factor receptor signaling pathway in cultured epidermal keratinocytes. Cell Growth Differ. 1994 Dec;5(12):1283–1292. [PubMed] [Google Scholar]
- Dominey A. M., Wang X. J., King L. E., Jr, Nanney L. B., Gagne T. A., Sellheyer K., Bundman D. S., Longley M. A., Rothnagel J. A., Greenhalgh D. A. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell Growth Differ. 1993 Dec;4(12):1071–1082. [PubMed] [Google Scholar]
- Gibbs S., Lohman F., Teubel W., van de Putte P., Backendorf C. Characterization of the human spr2 promoter: induction after UV irradiation or TPA treatment and regulation during differentiation of cultured primary keratinocytes. Nucleic Acids Res. 1990 Aug 11;18(15):4401–4407. doi: 10.1093/nar/18.15.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. B., Kulkarni A. B., Tennenbaum T., Hennings H., Flanders K. C., O'Reilly M., Sporn M. B., Karlsson S., Yuspa S. H. Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6076–6080. doi: 10.1073/pnas.90.13.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Basketter D. A., Couchman J. R., Rees D. A. Distribution and number of epidermal growth factor receptors in skin is related to epithelial cell growth. Dev Biol. 1983 Dec;100(2):506–512. doi: 10.1016/0012-1606(83)90243-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Couchman J. R. Distribution of epidermal growth factor receptors in rat tissues during embryonic skin development, hair formation, and the adult hair growth cycle. J Invest Dermatol. 1984 Aug;83(2):118–123. doi: 10.1111/1523-1747.ep12263298. [DOI] [PubMed] [Google Scholar]
- Hansen L. A., Tennant R. W. Follicular origin of epidermal papillomas in v-Ha-ras transgenic TG.AC mouse skin. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7822–7826. doi: 10.1073/pnas.91.16.7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck D. E., Laskin D. L., Gardner C. R., Laskin J. D. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing. J Biol Chem. 1992 Oct 25;267(30):21277–21280. [PubMed] [Google Scholar]
- Huh N., Kashiwagi M., Konishi C., Hashimoto Y., Kohno Y., Nomura S., Kuroki T. Isolation and characterization of a novel hair follicle-specific gene, Hacl-1. J Invest Dermatol. 1994 May;102(5):716–720. doi: 10.1111/1523-1747.ep12375385. [DOI] [PubMed] [Google Scholar]
- Kartasova T., Darwiche N., Kohno Y., Koizumi H., Osada S., Huh N., Lichti U., Steinert P. M., Kuroki T. Sequence and expression patterns of mouse SPR1: Correlation of expression with epithelial function. J Invest Dermatol. 1996 Feb;106(2):294–304. doi: 10.1111/1523-1747.ep12340741. [DOI] [PubMed] [Google Scholar]
- Kartasova T., van de Putte P. Isolation, characterization, and UV-stimulated expression of two families of genes encoding polypeptides of related structure in human epidermal keratinocytes. Mol Cell Biol. 1988 May;8(5):2195–2203. doi: 10.1128/mcb.8.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke N. C., Qiu T. H., Peiffer R. L., Oliver P., Smithies O., Lee D. C. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993 Apr 23;73(2):263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Mann G. B., Fowler K. J., Gabriel A., Nice E. C., Williams R. L., Dunn A. R. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993 Apr 23;73(2):249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Panaretto B. A., Carter N. B. Epidermal hyperplasia and wool follicle regression in sheep infused with epidermal growth factor. J Invest Dermatol. 1985 Mar;84(3):172–175. doi: 10.1111/1523-1747.ep12264699. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Panaretto B. A., Robertson D. Effects of epidermal growth factor on hair growth in the mouse. J Endocrinol. 1981 Feb;88(2):293–299. doi: 10.1677/joe.0.0880293. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Panaretto B. A., Robertson D. Epidermal growth factor delays the development of the epidermis and hair follicles of mice during growth of the first coat. Anat Rec. 1983 Jan;205(1):47–55. doi: 10.1002/ar.1092050107. [DOI] [PubMed] [Google Scholar]
- Morita K., Hogan M. E., Nanney L. B., King L. E., Jr, Manabe M., Sun T. T., Sundberg J. P. Cutaneous ultrastructural features of the flaky skin (fsn) mouse mutation. J Dermatol. 1995 Jun;22(6):385–395. doi: 10.1111/j.1346-8138.1995.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Murillas R., Larcher F., Conti C. J., Santos M., Ullrich A., Jorcano J. L. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J. 1995 Nov 1;14(21):5216–5223. doi: 10.1002/j.1460-2075.1995.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney L. B., Ellis D. L., Levine J., King L. E. Epidermal growth factor receptors in idiopathic and virally induced skin diseases. Am J Pathol. 1992 Apr;140(4):915–925. [PMC free article] [PubMed] [Google Scholar]
- Nanney L. B., Magid M., Stoscheck C. M., King L. E., Jr Comparison of epidermal growth factor binding and receptor distribution in normal human epidermis and epidermal appendages. J Invest Dermatol. 1984 Nov;83(5):385–393. doi: 10.1111/1523-1747.ep12264708. [DOI] [PubMed] [Google Scholar]
- Nanney L. B., Stoscheck C. M., King L. E., Jr, Underwood R. A., Holbrook K. A. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol. 1990 Jun;94(6):742–748. doi: 10.1111/1523-1747.ep12874601. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Teng C. T., Beckman W. C., Jr, Jefferson W. N., Hanson R. B., Miller J. V., McLachlan J. A. Fluctuations of lactoferrin protein and messenger ribonucleic acid in the reproductive tract of the mouse during the estrous cycle. Biol Reprod. 1992 Nov;47(5):903–915. doi: 10.1095/biolreprod47.5.903. [DOI] [PubMed] [Google Scholar]
- Paus R., Eichmüller S., Hofmann U., Czarnetzki B. M., Robinson P. Expression of classical and non-classical MHC class I antigens in murine hair follicles. Br J Dermatol. 1994 Aug;131(2):177–183. doi: 10.1111/j.1365-2133.1994.tb08488.x. [DOI] [PubMed] [Google Scholar]
- Pisansarakit P., du Cros D., Moore G. P. Cultivation of keratinocytes derived from epidermal explants of sheep skin and the roles of growth factors in the regulation of proliferation. Arch Dermatol Res. 1990;281(8):530–535. doi: 10.1007/BF00412739. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Cheng C. K., Titterington L., Meyers C. A., Stanley J. R., Steinert P. M., Yuspa S. H. Synthetic peptides corresponding to keratin subunits elicit highly specific antibodies. J Biol Chem. 1984 Jul 10;259(13):8037–8040. [PubMed] [Google Scholar]
- Rothnagel J. A., Roop D. R. Hair follicle companion layer: reacquainting an old friend. J Invest Dermatol. 1995 May;104(5 Suppl):42S–43S. doi: 10.1038/jid.1995.59. [DOI] [PubMed] [Google Scholar]
- Sibilia M., Wagner E. F. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995 Jul 14;269(5221):234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Threadgill D. W., Dlugosz A. A., Hansen L. A., Tennenbaum T., Lichti U., Yee D., LaMantia C., Mourton T., Herrup K., Harris R. C. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995 Jul 14;269(5221):230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Vassar R., Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991 May;5(5):714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Weinberg W. C., Goodman L. V., George C., Morgan D. L., Ledbetter S., Yuspa S. H., Lichti U. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol. 1993 Mar;100(3):229–236. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- Werner S., Peters K. G., Longaker M. T., Fuller-Pace F., Banda M. J., Williams L. T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate G. E., Craggs R. I., Gibson W. T. Immune privilege in hair growth. J Invest Dermatol. 1991 Sep;97(3):417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- Winter H., Siry P., Tobiasch E., Schweizer J. Sequence and expression of murine type I hair keratins mHa2 and mHa3. Exp Cell Res. 1994 Jun;212(2):190–200. doi: 10.1006/excr.1994.1134. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Viguera C., Nims R. Maintenance of human skin on nude mice for studies of chemical carcinogenesis. Cancer Lett. 1979 Apr;6(4-5):301–310. doi: 10.1016/s0304-3835(79)80049-x. [DOI] [PubMed] [Google Scholar]
- du Cros D. L., Isaacs K., Moore G. P. Localization of epidermal growth factor immunoreactivity in sheep skin during wool follicle development. J Invest Dermatol. 1992 Jan;98(1):109–115. doi: 10.1111/1523-1747.ep12496010. [DOI] [PubMed] [Google Scholar]