Abstract
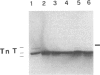
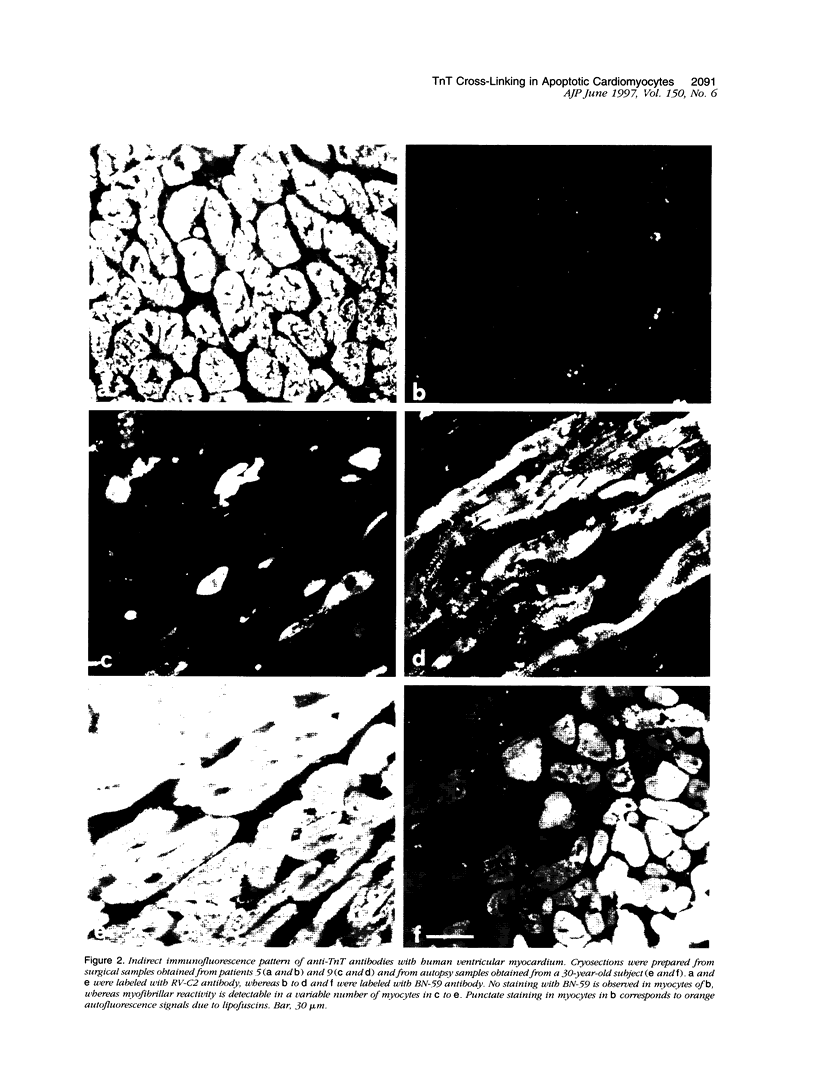
Intracellular calcium overload of guinea pig cardiomyocytes is accompanied by troponin T cross-linking, which is revealed by changes in immunoreactivity of anti-troponin T antibodies. We presently investigated whether the same process is detectable in the human heart. Immunohistochemistry shows myofibrillar staining with BN-59 anti-troponin T antibody with rare cardiomyocytes in samples obtained at surgery, whereas approximately 50% of myocytes are labeled in heart samples taken at autopsy within 3 hours of death, and every cardiomyocyte is stained after exposure of biopsy sections to 10 mmol/L calcium. Western blot analysis shows reactive polypeptides of approximately 70 and 85 to 90 kd in addition to troponin T in both treated and autopsy heart sections. Neither reactivity in immunohistochemistry nor additional reactive polypeptides in Western blot are detectable when calpain or transglutaminase is inhibited during exposure of sections to high calcium. Troponin T crosslinking occurs also in isolated myofibrils, which show staining with BN-59 at either sarcomeric A or I bands. Labeling with TdT-mediated dUTP nick and labeling (TUNEL) to demonstrate apoptosis reveals DNA fragmentation in BN-59-positive myocytes. Thus, troponin T cross-linking occurs in human cardiac myocytes concomitantly with apoptosis and autopsy autolysis, suggesting that similar cytosolic alterations can be produced by different types of myocyte death.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. A., Greig A., Mark T. M., Malouf N. N., Oakeley A. E., Ungerleider R. M., Allen P. D., Kay B. K. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res. 1995 Apr;76(4):681–686. doi: 10.1161/01.res.76.4.681. [DOI] [PubMed] [Google Scholar]
- Anderson P. A., Oakeley A. E. Immunological identification of five troponin T isoforms reveals an elaborate maturational troponin T profile in rabbit myocardium. Circ Res. 1989 Oct;65(4):1087–1093. doi: 10.1161/01.res.65.4.1087. [DOI] [PubMed] [Google Scholar]
- Azzarello G., Sartore S., Saggin L., Gorza L., D'Andrea E., Chieco-Bianchi L., Schiaffino S. Myosin isoform expression in rat rhabdomyosarcoma induced by Moloney murine sarcoma virus. J Cancer Res Clin Oncol. 1987;113(5):417–429. doi: 10.1007/BF00390035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbato R., Menabò R., Dainese P., Carafoli E., Schiaffino S., Di Lisa F. Binding of cytosolic proteins to myofibrils in ischemic rat hearts. Circ Res. 1996 May;78(5):821–828. doi: 10.1161/01.res.78.5.821. [DOI] [PubMed] [Google Scholar]
- Baudet S., Kuznetsov A., Merciai N., Gorza L., Ventura-Clapier R. Biochemical, mechanical and energetic characterization of right ventricular hypertrophy in the ferret heart. J Mol Cell Cardiol. 1994 Dec;26(12):1573–1586. doi: 10.1006/jmcc.1994.1177. [DOI] [PubMed] [Google Scholar]
- Bing O. H. Hypothesis: apoptosis may be a mechanism for the transition to heart failure with chronic pressure overload. J Mol Cell Cardiol. 1994 Aug;26(8):943–948. doi: 10.1006/jmcc.1994.1115. [DOI] [PubMed] [Google Scholar]
- Clark W. A. Evidence for post-translational kinetic compartmentation of protein turnover pools in isolated adult cardiac myocytes. J Biol Chem. 1993 Sep 25;268(27):20243–20251. [PubMed] [Google Scholar]
- Fesus L., Madi A., Balajthy Z., Nemes Z., Szondy Z. Transglutaminase induction by various cell death and apoptosis pathways. Experientia. 1996 Oct 31;52(10-11):942–949. doi: 10.1007/BF01920102. [DOI] [PubMed] [Google Scholar]
- Gold R., Schmied M., Giegerich G., Breitschopf H., Hartung H. P., Toyka K. V., Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994 Aug;71(2):219–225. [PubMed] [Google Scholar]
- Goldfine S. M., Einheber S., Fischman D. A. Cell-free incorporation of newly synthesized myosin subunits into thick myofilaments. J Muscle Res Cell Motil. 1991 Apr;12(2):161–170. doi: 10.1007/BF01774035. [DOI] [PubMed] [Google Scholar]
- Gorza L., Menabò R., Vitadello M., Bergamini C. M., Di Lisa F. Cardiomyocyte troponin T immunoreactivity is modified by cross-linking resulting from intracellular calcium overload. Circulation. 1996 May 15;93(10):1896–1904. doi: 10.1161/01.cir.93.10.1896. [DOI] [PubMed] [Google Scholar]
- Gorza L., Mercadier J. J., Schwartz K., Thornell L. E., Sartore S., Schiaffino S. Myosin types in the human heart. An immunofluorescence study of normal and hypertrophied atrial and ventricular myocardium. Circ Res. 1984 Jun;54(6):694–702. doi: 10.1161/01.res.54.6.694. [DOI] [PubMed] [Google Scholar]
- Greenberg C. S., Birckbichler P. J., Rice R. H. Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991 Dec;5(15):3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W., Bläuenstein I. E., Eppenberger H. M. Comparison of the localization of several muscle proteins in relaxed and contracted myofibrils. Experientia. 1978 Jan 15;34(1):38–40. doi: 10.1007/BF01921887. [DOI] [PubMed] [Google Scholar]
- Inomata M., Saito Y., Kon K., Kawashima S. Binding sites for calcium-activated neutral protease on erythrocyte membranes are not membrane phospholipids. Biochem Biophys Res Commun. 1990 Sep 14;171(2):625–632. doi: 10.1016/0006-291x(90)91192-u. [DOI] [PubMed] [Google Scholar]
- Iwai N., Shimoike H., Kinoshita M. Genes up-regulated in hypertrophied ventricle. Biochem Biophys Res Commun. 1995 Apr 17;209(2):527–534. doi: 10.1006/bbrc.1995.1533. [DOI] [PubMed] [Google Scholar]
- Kang S. J., Shin K. S., Song W. K., Ha D. B., Chung C. H., Kang M. S. Involvement of transglutaminase in myofibril assembly of chick embryonic myoblasts in culture. J Cell Biol. 1995 Sep;130(5):1127–1136. doi: 10.1083/jcb.130.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katus H. A., Remppis A., Neumann F. J., Scheffold T., Diederich K. W., Vinar G., Noe A., Matern G., Kuebler W. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991 Mar;83(3):902–912. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Calcium hypothesis of Alzheimer's disease and brain aging. Ann N Y Acad Sci. 1994 Dec 15;747:1–11. doi: 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- Mesnard L., Logeart D., Taviaux S., Diriong S., Mercadier J. J., Samson F. Human cardiac troponin T: cloning and expression of new isoforms in the normal and failing heart. Circ Res. 1995 Apr;76(4):687–692. doi: 10.1161/01.res.76.4.687. [DOI] [PubMed] [Google Scholar]
- Murachi T. Intracellular regulatory system involving calpain and calpastatin. Biochem Int. 1989 Feb;18(2):263–294. [PubMed] [Google Scholar]
- Nakaoka H., Perez D. M., Baek K. J., Das T., Husain A., Misono K., Im M. J., Graham R. M. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science. 1994 Jun 10;264(5165):1593–1596. doi: 10.1126/science.7911253. [DOI] [PubMed] [Google Scholar]
- Remppis A., Scheffold T., Greten J., Haass M., Greten T., Kübler W., Katus H. A. Intracellular compartmentation of troponin T: release kinetics after global ischemia and calcium paradox in the isolated perfused rat heart. J Mol Cell Cardiol. 1995 Feb;27(2):793–803. doi: 10.1016/0022-2828(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Saggin L., Ausoni S., Gorza L., Sartore S., Schiaffino S. Troponin T switching in the developing rat heart. J Biol Chem. 1988 Dec 5;263(34):18488–18492. [PubMed] [Google Scholar]
- Saggin L., Gorza L., Ausoni S., Schiaffino S. Cardiac troponin T in developing, regenerating and denervated rat skeletal muscle. Development. 1990 Oct;110(2):547–554. doi: 10.1242/dev.110.2.547. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Sorimachi H., Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994 Aug;8(11):814–822. [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Sharov V. G., Sabbah H. N., Shimoyama H., Goussev A. V., Lesch M., Goldstein S. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol. 1996 Jan;148(1):141–149. [PMC free article] [PubMed] [Google Scholar]
- Spencer M. J., Croall D. E., Tidball J. G. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995 May 5;270(18):10909–10914. doi: 10.1074/jbc.270.18.10909. [DOI] [PubMed] [Google Scholar]
- Squìer M. K., Miller A. C., Malkinson A. M., Cohen J. J. Calpain activation in apoptosis. J Cell Physiol. 1994 May;159(2):229–237. doi: 10.1002/jcp.1041590206. [DOI] [PubMed] [Google Scholar]
- Umansky S. R., Cuenco G. M., Khutzian S. S., Barr P. J., Tomei L. D. Post-ischemic apoptotic death of rat neonatal cardiomyocytes. Cell Death Differ. 1995 Oct;2(4):235–241. [PubMed] [Google Scholar]
- Vaux D. L., Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]