Abstract
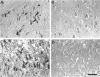
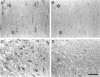
In this study, we demonstrate that two important regulators of the cell cycle, cyclin-dependent kinase-4 and its inhibitor p16, are increased in the brains of cases of Alzheimer's disease patients compared with age-matched controls. Both proteins are increased in the pyramidal neurons of the hippocampus, including those neurons containing neurofibrillary tangles and granulovacuolar degeneration. As p16 is not normally found in terminally differentiated neurons, it seems paradoxical that it is increased in Alzheimer's disease unless it is responding to increases in cyclin-dependent kinase-4 or other cell cycle regulators. Induction of the latter, a protein that signals re-entry and progression through the cell cycle, may itself be the consequence of alpha response to a growth stimulus. Re-entry into the cell cycle is likely deleterious in terminally differentiated neurons and may contribute to the biochemical abnormalities, such as oxidative stress and hyperphosphorylated tau protein, as well as the neuronal degeneration characteristic of the pathology of Alzheimer's disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann K., Mandelkow E. M., Biernat J., Piwnica-Worms H., Mandelkow E. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993 Dec 28;336(3):417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- Berry D. E., Lu Y., Schmidt B., Fallon P. G., O'Connell C., Hu S. X., Xu H. J., Blanck G. Retinoblastoma protein inhibits IFN-gamma induced apoptosis. Oncogene. 1996 Apr 18;12(8):1809–1819. [PubMed] [Google Scholar]
- Bramblett G. T., Goedert M., Jakes R., Merrick S. E., Trojanowski J. Q., Lee V. M. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993 Jun;10(6):1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Brion J. P., Octave J. N., Couck A. M. Distribution of the phosphorylated microtubule-associated protein tau in developing cortical neurons. Neuroscience. 1994 Dec;63(3):895–909. doi: 10.1016/0306-4522(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Crutcher K. A., Scott S. A., Liang S., Everson W. V., Weingartner J. Detection of NGF-like activity in human brain tissue: increased levels in Alzheimer's disease. J Neurosci. 1993 Jun;13(6):2540–2550. doi: 10.1523/JNEUROSCI.13-06-02540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio F., Ceriello A. Decreased cultured endothelial cell proliferation in high glucose medium is reversed by antioxidants: new insights on the pathophysiological mechanisms of diabetic vascular complications. In Vitro Cell Dev Biol. 1992 Nov-Dec;28A(11-12):787–790. doi: 10.1007/BF02631069. [DOI] [PubMed] [Google Scholar]
- Dobashi Y., Kudoh T., Toyoshima K., Akiyama T. Persistent activation of CDK4 during neuronal differentiation of rat pheochromocytoma PC12 cells. Biochem Biophys Res Commun. 1996 Apr 16;221(2):351–355. doi: 10.1006/bbrc.1996.0599. [DOI] [PubMed] [Google Scholar]
- Donaldson K. L., Goolsby G. L., Kiener P. A., Wahl A. F. Activation of p34cdc2 coincident with taxol-induced apoptosis. Cell Growth Differ. 1994 Oct;5(10):1041–1050. [PubMed] [Google Scholar]
- Elledge S. J., Winston J., Harper J. W. A question of balance: the role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 1996 Oct;6(10):388–392. doi: 10.1016/0962-8924(96)10030-1. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Yan C. Y., Greene L. A. N-acetylcysteine (D- and L-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci. 1995 Apr;15(4):2857–2866. doi: 10.1523/JNEUROSCI.15-04-02857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. S., Estus S., Johnson E. M., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994 Feb;12(2):343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Six J., Lübke U., Vandermeeren M., Cras P., Trojanowski J. Q., Lee V. M. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4051–4055. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pinilla F., Cummings B. J., Cotman C. W. Induction of basic fibroblast growth factor in Alzheimer's disease pathology. Neuroreport. 1990 Nov-Dec;1(3-4):211–214. doi: 10.1097/00001756-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Kanemaru K., Takio K., Miura R., Titani K., Ihara Y. Fetal-type phosphorylation of the tau in paired helical filaments. J Neurochem. 1992 May;58(5):1667–1675. doi: 10.1111/j.1471-4159.1992.tb10039.x. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Khleif S. N., DeGregori J., Yee C. L., Otterson G. A., Kaye F. J., Nevins J. R., Howley P. M. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4350–4354. doi: 10.1073/pnas.93.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiess M., Gill R. M., Hamel P. A. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene. 1995 Jan 5;10(1):159–166. [PubMed] [Google Scholar]
- Kranenburg O., de Groot R. P., Van der Eb A. J., Zantema A. Differentiation of P19 EC cells leads to differential modulation of cyclin-dependent kinase activities and to changes in the cell cycle profile. Oncogene. 1995 Jan 5;10(1):87–95. [PubMed] [Google Scholar]
- Kranenburg O., van der Eb A. J., Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996 Jan 2;15(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- Ledesma M. D., Correas I., Avila J., Díaz-Nido J. Implication of brain cdc2 and MAP2 kinases in the phosphorylation of tau protein in Alzheimer's disease. FEBS Lett. 1992 Aug 17;308(2):218–224. doi: 10.1016/0014-5793(92)81278-t. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Goedert M., Hill W. D., Lee V. M., Trojanowski J. Q. Tau proteins are abnormally expressed in olfactory epithelium of Alzheimer patients and developmentally regulated in human fetal spinal cord. Exp Neurol. 1993 May;121(1):93–105. doi: 10.1006/exnr.1993.1074. [DOI] [PubMed] [Google Scholar]
- Lee P. J., Alam J., Wiegand G. W., Choi A. M. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J., Huang Q. Q., Qi Z., Winkfein R. J., Aebersold R., Hunt T., Wang J. H. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994 Sep 29;371(6496):423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- Liu W. K., Williams R. T., Hall F. L., Dickson D. W., Yen S. H. Detection of a Cdc2-related kinase associated with Alzheimer paired helical filaments. Am J Pathol. 1995 Jan;146(1):228–238. [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Alford M., Hansen L. A., Saitoh T. Immunoreactivity of the nuclear antigen p105 is associated with plaques and tangles in Alzheimer's disease. Lab Invest. 1993 Nov;69(5):562–569. [PubMed] [Google Scholar]
- Olson L. NGF and the treatment of Alzheimer's disease. Exp Neurol. 1993 Nov;124(1):5–15. doi: 10.1006/exnr.1993.1167. [DOI] [PubMed] [Google Scholar]
- Perry G., Siedlak S. L., Richey P., Kawai M., Cras P., Kalaria R. N., Galloway P. G., Scardina J. M., Cordell B., Greenberg B. D. Association of heparan sulfate proteoglycan with the neurofibrillary tangles of Alzheimer's disease. J Neurosci. 1991 Nov;11(11):3679–3683. doi: 10.1523/JNEUROSCI.11-11-03679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope W. B., Lambert M. P., Leypold B., Seupaul R., Sletten L., Krafft G., Klein W. L. Microtubule-associated protein tau is hyperphosphorylated during mitosis in the human neuroblastoma cell line SH-SY5Y. Exp Neurol. 1994 Apr;126(2):185–194. doi: 10.1006/exnr.1994.1057. [DOI] [PubMed] [Google Scholar]
- Pope W., Enam S. A., Bawa N., Miller B. E., Ghanbari H. A., Klein W. L. Phosphorylated tau epitope of Alzheimer's disease is coupled to axon development in the avian central nervous system. Exp Neurol. 1993 Mar;120(1):106–113. doi: 10.1006/exnr.1993.1044. [DOI] [PubMed] [Google Scholar]
- Premkumar D. R., Smith M. A., Richey P. L., Petersen R. B., Castellani R., Kutty R. K., Wiggert B., Perry G., Kalaria R. N. Induction of heme oxygenase-1 mRNA and protein in neocortex and cerebral vessels in Alzheimer's disease. J Neurochem. 1995 Sep;65(3):1399–1402. doi: 10.1046/j.1471-4159.1995.65031399.x. [DOI] [PubMed] [Google Scholar]
- Preuss U., Döring F., Illenberger S., Mandelkow E. M. Cell cycle-dependent phosphorylation and microtubule binding of tau protein stably transfected into Chinese hamster ovary cells. Mol Biol Cell. 1995 Oct;6(10):1397–1410. doi: 10.1091/mbc.6.10.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schang L. M., Hossain A., Jones C. The latency-related gene of bovine herpesvirus 1 encodes a product which inhibits cell cycle progression. J Virol. 1996 Jun;70(6):3807–3814. doi: 10.1128/jvi.70.6.3807-3814.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. D-type cyclins. Trends Biochem Sci. 1995 May;20(5):187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Kutty R. K., Richey P. L., Yan S. D., Stern D., Chader G. J., Wiggert B., Petersen R. B., Perry G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. Am J Pathol. 1994 Jul;145(1):42–47. [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Sayre L. M., Monnier V. M., Perry G. Radical AGEing in Alzheimer's disease. Trends Neurosci. 1995 Apr;18(4):172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- Smith T. W., Lippa C. F. Ki-67 immunoreactivity in Alzheimer's disease and other neurodegenerative disorders. J Neuropathol Exp Neurol. 1995 May;54(3):297–303. doi: 10.1097/00005072-199505000-00002. [DOI] [PubMed] [Google Scholar]
- Spana C., O'Rourke E. C., Bolen J. B., Fargnoli J. Analysis of the tyrosine protein kinase p56lck expressed as a glutathione S-transferase fusion protein in Spodoptera frugiperda cells. Protein Expr Purif. 1993 Oct;4(5):390–397. doi: 10.1006/prep.1993.1051. [DOI] [PubMed] [Google Scholar]
- Stewart B. W. Mechanisms of apoptosis: integration of genetic, biochemical, and cellular indicators. J Natl Cancer Inst. 1994 Sep 7;86(17):1286–1296. doi: 10.1093/jnci/86.17.1286. [DOI] [PubMed] [Google Scholar]
- Stopa E. G., Gonzalez A. M., Chorsky R., Corona R. J., Alvarez J., Bird E. D., Baird A. Basic fibroblast growth factor in Alzheimer's disease. Biochem Biophys Res Commun. 1990 Sep 14;171(2):690–696. doi: 10.1016/0006-291x(90)91201-3. [DOI] [PubMed] [Google Scholar]
- Sugano T., Nitta M., Ohmori H., Yamaizumi M. Nuclear accumulation of p53 in normal human fibroblasts is induced by various cellular stresses which evoke the heat shock response, independently of the cell cycle. Jpn J Cancer Res. 1995 May;86(5):415–418. doi: 10.1111/j.1349-7006.1995.tb03072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Oishi M., Marshak D. R., Czernik A. J., Nairn A. C., Greengard P. Cell cycle-dependent regulation of the phosphorylation and metabolism of the Alzheimer amyloid precursor protein. EMBO J. 1994 Mar 1;13(5):1114–1122. doi: 10.1002/j.1460-2075.1994.tb06360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y., Tatsuka M., Jinno S., Okayama H. Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet irradiation. Nature. 1995 Jul 27;376(6538):358–362. doi: 10.1038/376358a0. [DOI] [PubMed] [Google Scholar]
- Vincent I., Rosado M., Davies P. Mitotic mechanisms in Alzheimer's disease? J Cell Biol. 1996 Feb;132(3):413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhide C. C., Van Dang C., Dipersio J., Kenedy A. A., Bray P. F. Overexpression of cyclin D1 in the Dami megakaryocytic cell line causes growth arrest. Blood. 1995 Jul 1;86(1):294–304. [PubMed] [Google Scholar]
- Williams M. E., Swerdlow S. H. Cyclin D1 overexpression in non-Hodgkin's lymphoma with chromosome 11 bcl-1 rearrangement. Ann Oncol. 1994;5 (Suppl 1):71–73. doi: 10.1093/annonc/5.suppl_1.s71. [DOI] [PubMed] [Google Scholar]
- Yan G. Z., Ziff E. B. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995 Sep;15(9):6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal E. A., Gómez-Pinilla F., Cotman C. W. Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport. 1993 Jan;4(1):69–72. doi: 10.1097/00001756-199301000-00018. [DOI] [PubMed] [Google Scholar]