Abstract
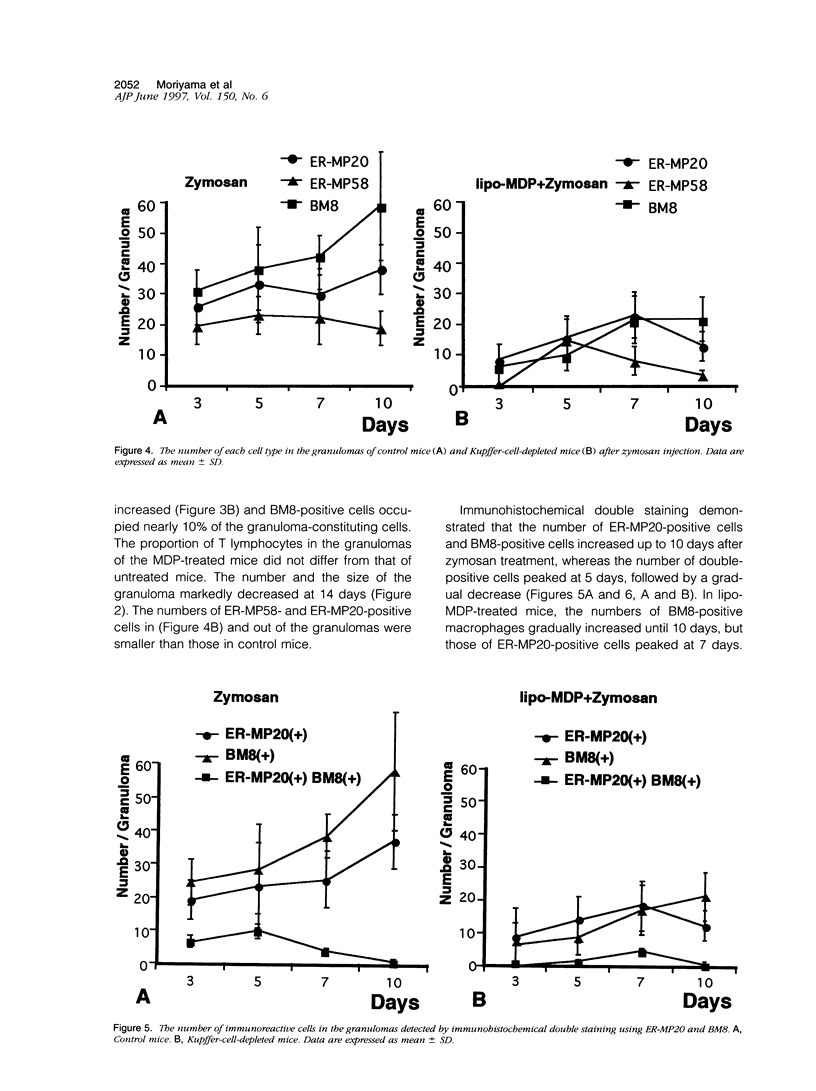
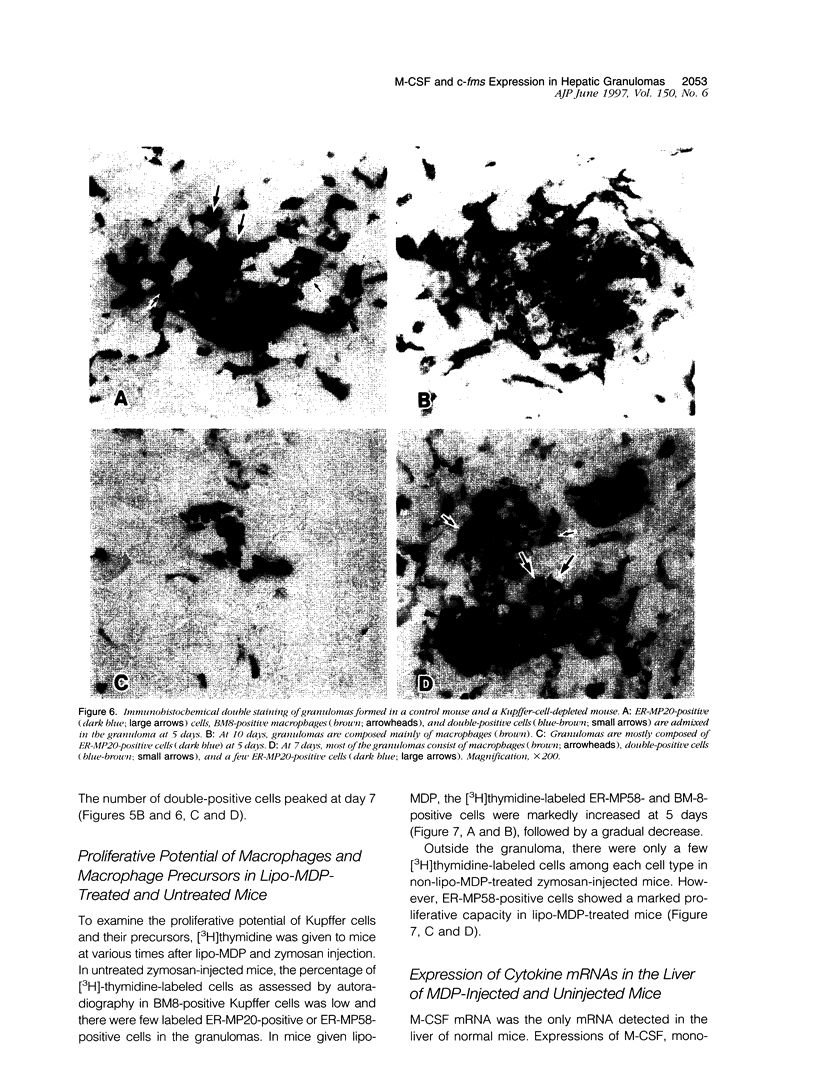
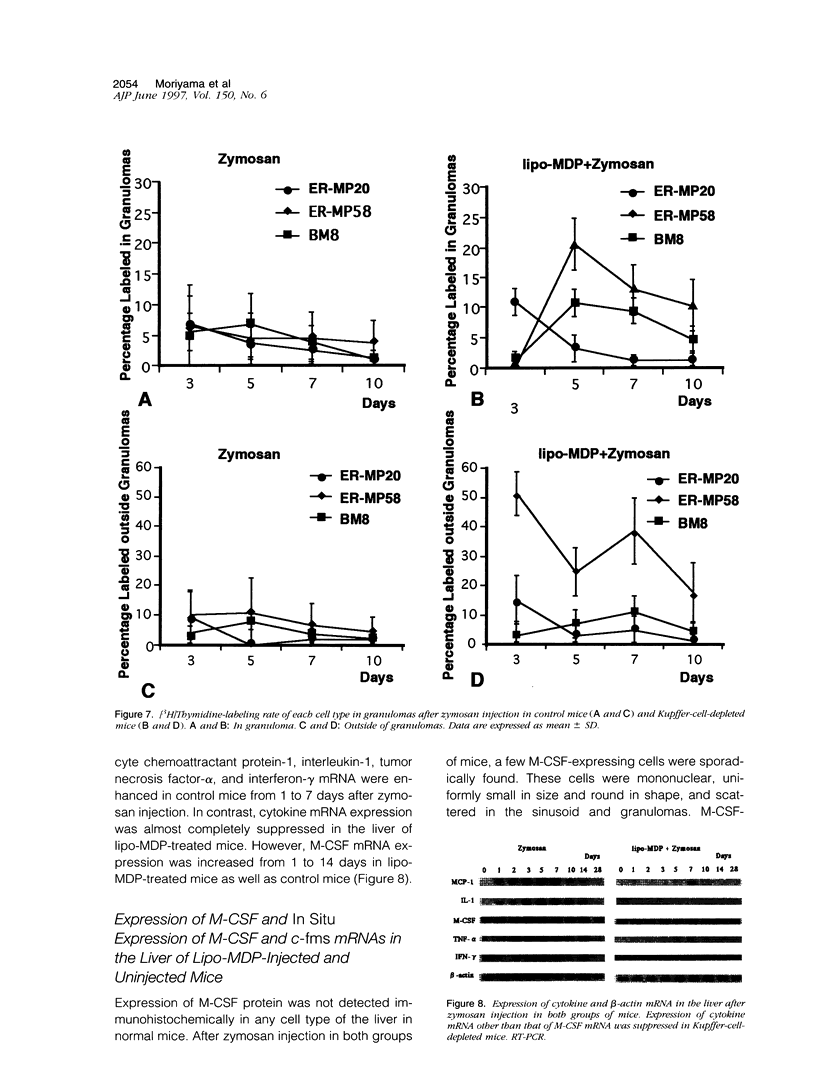
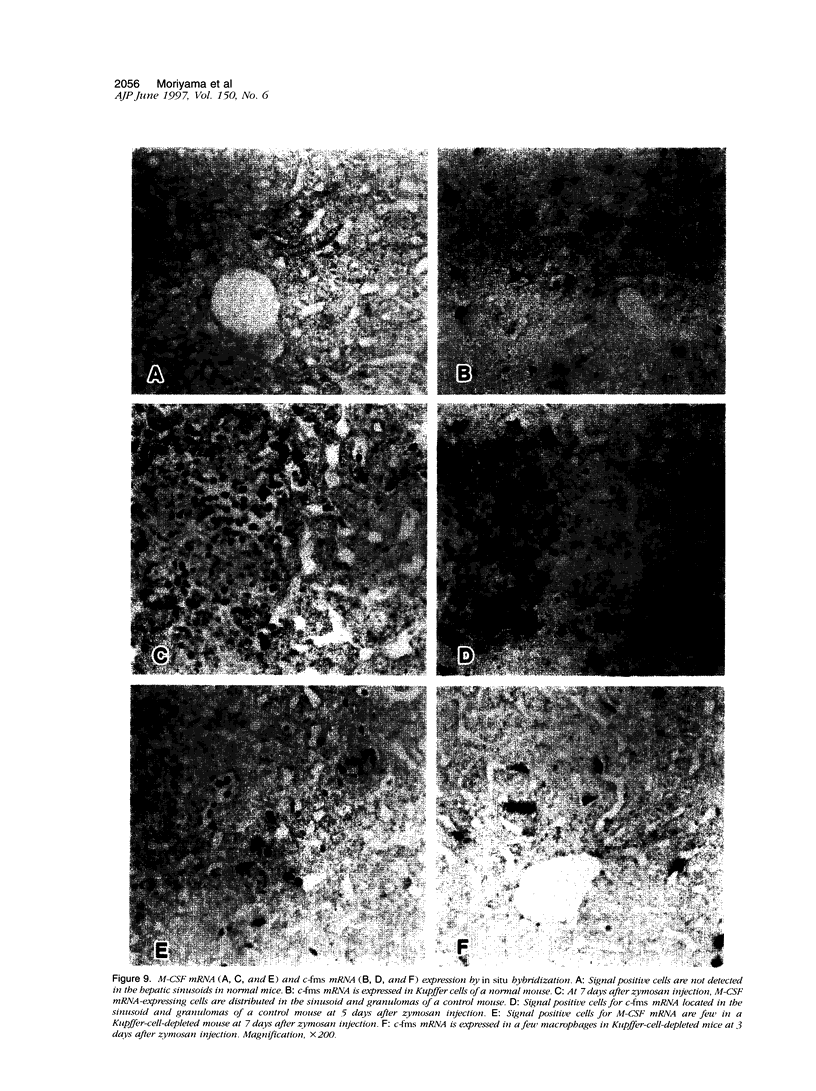
In mice administered with liposome-entrapped dichloromethylene diphosphonate, which depletes Kupffer cells, the size and the number of zymosan-induced granulomas in the liver were smaller than in untreated mice. The number of macrophage precursors, as detected by the monoclonal antibodies for macrophage precursors, increased after zymosan injection in both groups of mice, proliferated, and differentiated into macrophages. Expression of macrophage colony-stimulating factor (M-CSF), interleukin-1, monocyte chemoattractant protein-1, tumor necrosis factor-alpha, and interferon-gamma mRNA was enhanced in the stage of granuloma formation in the control mouse liver, whereas it was suppressed in Kupffer-cell-depleted mice. However, M-CSF mRNA expression was increased in the Kupffer-cell-depleted mice to form granulomas in the late stages. In situ hybridization demonstrated the expression of M-CSF mRNA and c-fms mRNA in Kupffer cells and monocyte-derived macrophages in the sinusoid and granulomas. The concentration of M-CSF in serum of zymosan-injected control mice was within normal range, but that of zymosan-treated or untreated Kupffer-cell-depleted mice was markedly elevated at day 1. These findings imply that Kupffer cells are indispensable for granuloma formation and produce various cytokines including M-CSF. The local production and consumption of M-CSF in the liver may play a crucial role in granulomatous inflammation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceci R. J., Shanahan F., Stanley E. R., Pollard J. W. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay M., Webb C. G., Sachs L. Control of hematopoietic cell growth regulators during mouse fetal development. Mol Cell Biol. 1987 Sep;7(9):3361–3364. doi: 10.1128/mcb.7.9.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci A., Pollard J. W., Stanley E. R. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986 Sep 1;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
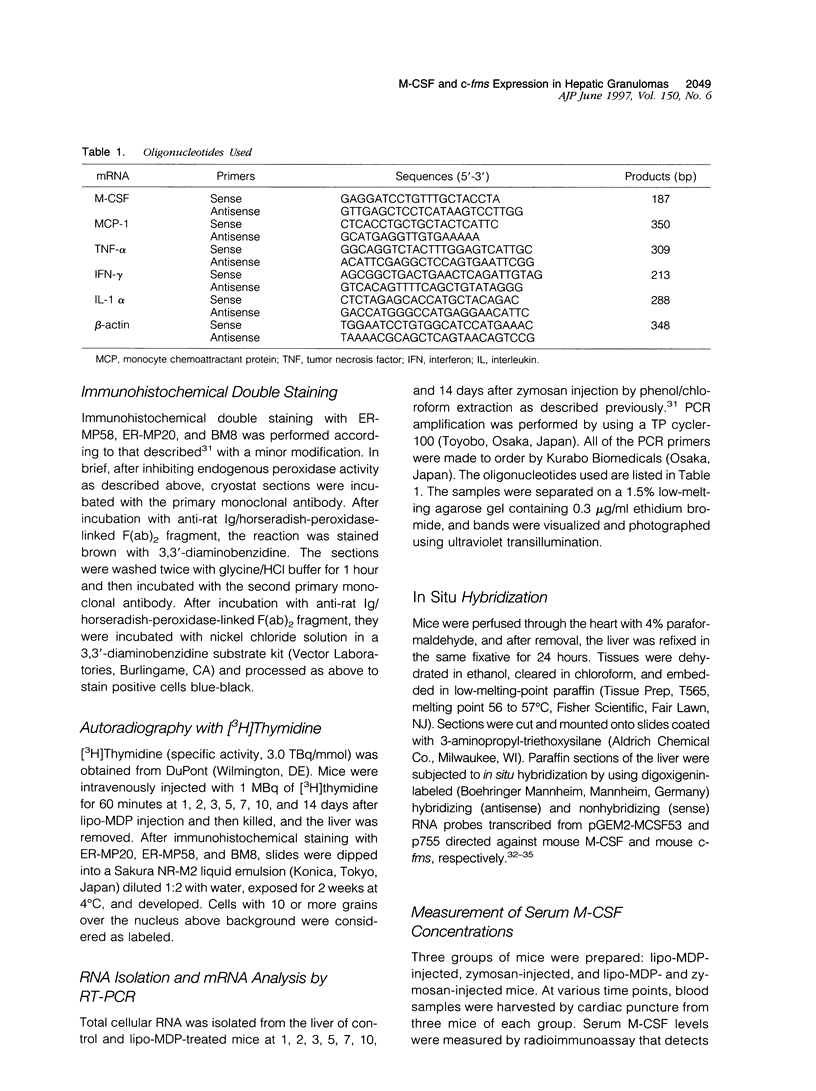
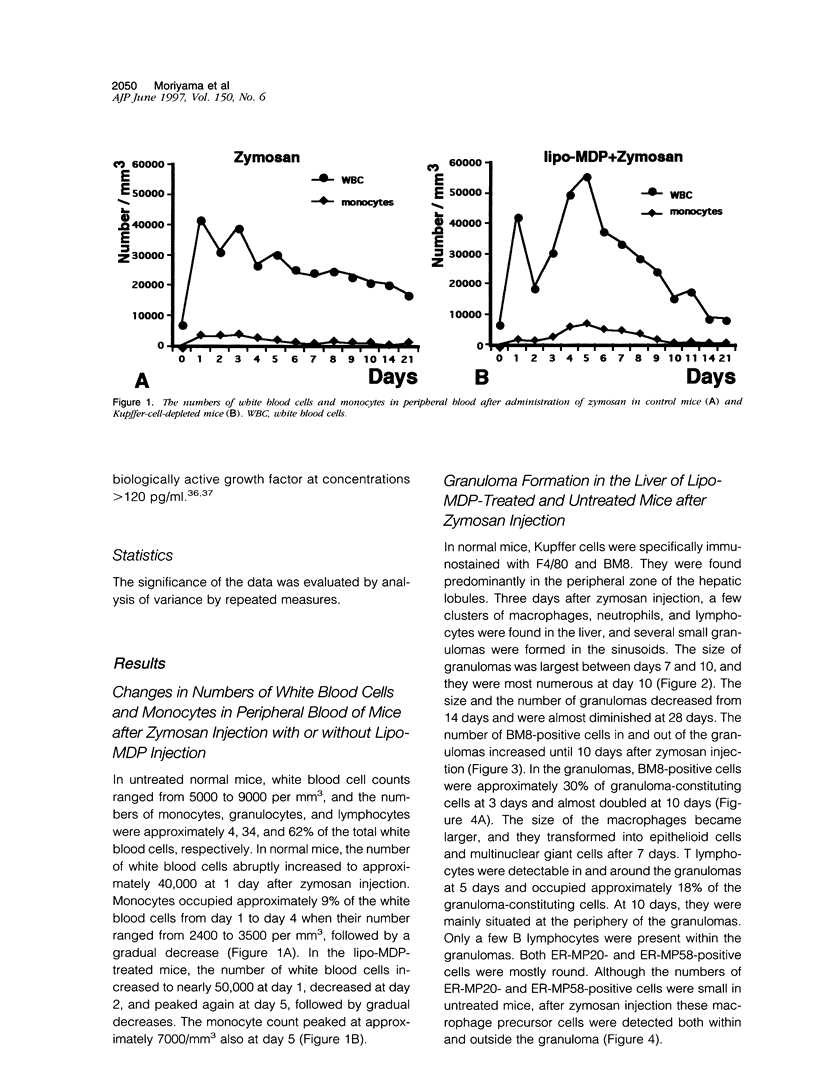
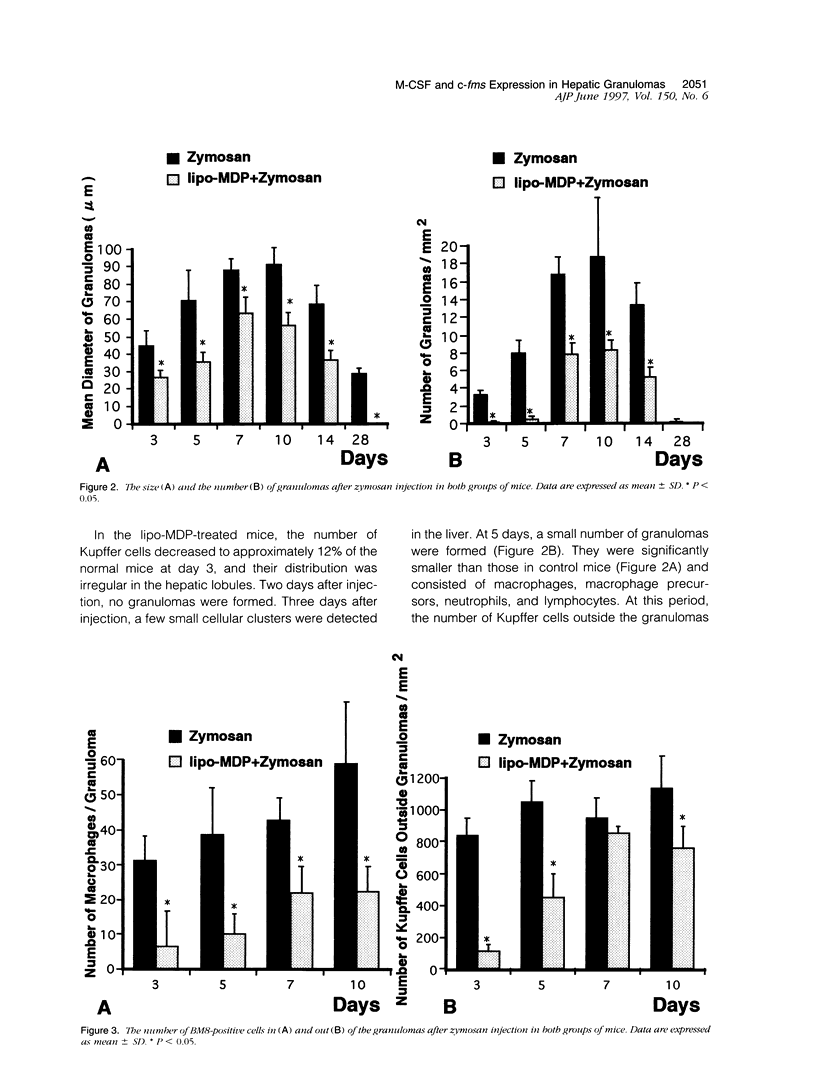
- Bouwens L., Wisse E. Proliferation, kinetics, and fate of monocytes in rat liver during a zymosan-induced inflammation. J Leukoc Biol. 1985 May;37(5):531–543. doi: 10.1002/jlb.37.5.531. [DOI] [PubMed] [Google Scholar]
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg K., Tapley P., Haystead C., Rohrschneider L. The role of kinase activity and the kinase insert region in ligand-induced internalization and degradation of the c-fms protein. EMBO J. 1991 Apr;10(4):877–883. doi: 10.1002/j.1460-2075.1991.tb08020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini M. G., Dominguez M. G., Mocci S., Wetterwald A., Felix R., Fleisch H., Chisholm O., Hofstetter W., Pollard J. W., Stanley E. R. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994 Jun;120(6):1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Kay J. Isolation and characterization of beta-glucan receptors on human mononuclear phagocytes. J Exp Med. 1991 Jun 1;173(6):1511–1520. doi: 10.1084/jem.173.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Hepatic granulomas induced by glucan. An ultrastructural and peroxidase-cytochemical study. Lab Invest. 1980 Aug;43(2):172–181. [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Induction of focal hemopoiesis in adult rat liver by glucan, a macrophage activator. A cytochemical and ultrastructural study. Lab Invest. 1980 Feb;42(2):217–224. [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. The appearance of transition forms between monocytes and Kupffer cells in the liver of rats treated with glucan. A cytochemical and ultrastructural study. J Exp Med. 1979 Apr 1;149(4):883–897. doi: 10.1084/jem.149.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delemarre F. G., Kors N., Kraal G., van Rooijen N. Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J Leukoc Biol. 1990 Mar;47(3):251–257. doi: 10.1002/jlb.47.3.251. [DOI] [PubMed] [Google Scholar]
- Hofstetter W., Wetterwald A., Cecchini M. G., Mueller C., Felix R. Detection of transcripts and binding sites for colony-stimulating factor-1 during bone development. Bone. 1995 Aug;17(2):145–151. doi: 10.1016/s8756-3282(95)00163-8. [DOI] [PubMed] [Google Scholar]
- Honda Y., Takahashi K., Naito M., Fujiyama S. The role of macrophage colony-stimulating factor in the differentiation and proliferation of Kupffer cells in the liver of protein-deprived mice. Lab Invest. 1995 Jun;72(6):696–706. [PubMed] [Google Scholar]
- Kiwada H., Niimura H., Fujisaki Y., Yamada S., Kato Y. Application of synthetic alkyl glycoside vesicles as drug carriers. I. Preparation and physical properties. Chem Pharm Bull (Tokyo) 1985 Feb;33(2):753–759. doi: 10.1248/cpb.33.753. [DOI] [PubMed] [Google Scholar]
- Ladner M. B., Martin G. A., Noble J. A., Nikoloff D. M., Tal R., Kawasaki E. S., White T. J. Human CSF-1: gene structure and alternative splicing of mRNA precursors. EMBO J. 1987 Sep;6(9):2693–2698. doi: 10.1002/j.1460-2075.1987.tb02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenen P. J., Melis M., Slieker W. A., Van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol. 1990 Jan;20(1):27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- Leenen P. J., de Bruijn M. F., Voerman J. S., Campbell P. A., van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994 Sep 14;174(1-2):5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Li W., Stanley E. R. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991 Feb;10(2):277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka Y., Naito M., Sato T., Takahashi K. Immunophenotypic and ultrastructural heterogeneity of macrophage differentiation in bone marrow and fetal hematopoiesis of mouse in vitro and in vivo. J Leukoc Biol. 1994 May;55(5):642–651. doi: 10.1002/jlb.55.5.642. [DOI] [PubMed] [Google Scholar]
- Naito M., Hayashi S., Yoshida H., Nishikawa S., Shultz L. D., Takahashi K. Abnormal differentiation of tissue macrophage populations in 'osteopetrosis' (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991 Sep;139(3):657–667. [PMC free article] [PubMed] [Google Scholar]
- Naito M., Nagai H., Kawano S., Umezu H., Zhu H., Moriyama H., Yamamoto T., Takatsuka H., Takei Y. Liposome-encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J Leukoc Biol. 1996 Sep;60(3):337–344. doi: 10.1002/jlb.60.3.337. [DOI] [PubMed] [Google Scholar]
- Naito M., Takahashi K. The role of Kupffer cells in glucan-induced granuloma formation in the liver of mice depleted of blood monocytes by administration of strontium-89. Lab Invest. 1991 May;64(5):664–674. [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Hunt J. S., Wiktor-Jedrzejczak W., Stanley E. R. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991 Nov;148(1):273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- Regenstreif L. J., Rossant J. Expression of the c-fms proto-oncogene and of the cytokine, CSF-1, during mouse embryogenesis. Dev Biol. 1989 May;133(1):284–294. doi: 10.1016/0012-1606(89)90319-9. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Myones B. L., Newman S. L., Lachmann P. J. Specificity of membrane complement receptor type three (CR3) for beta-glucans. Complement. 1987;4(2):61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Mabuchi A., Kuriya S., Sudo T., Aida T., Asano G., Shoji T., Yokomuro K. Production of granulocyte-macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes). Reg Immunol. 1990;3(5):260–267. [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Naito M., Umeda S., Shultz L. D. The role of macrophage colony-stimulating factor in hepatic glucan-induced granuloma formation in the osteopetrosis mutant mouse defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1994 Jun;144(6):1381–1392. [PMC free article] [PubMed] [Google Scholar]
- Tsukui T., Kikuchi K., Mabuchi A., Sudo T., Sakamoto T., Sato N., Tsuneoka K., Shikita M., Aida T., Asano G. Production of macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes). J Leukoc Biol. 1992 Oct;52(4):383–389. doi: 10.1002/jlb.52.4.383. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Kors N., vd Ende M., Dijkstra C. D. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990 May;260(2):215–222. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994 Sep 14;174(1-2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989 Nov 13;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- Webb S. E., Pollard J. W., Jones G. E. Direct observation and quantification of macrophage chemoattraction to the growth factor CSF-1. J Cell Sci. 1996 Apr;109(Pt 4):793–803. doi: 10.1242/jcs.109.4.793. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Naito M., Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukoc Biol. 1990 Mar;47(3):195–205. [PubMed] [Google Scholar]
- Yamamoto T., Naito M., Moriyama H., Umezu H., Matsuo H., Kiwada H., Arakawa M. Repopulation of murine Kupffer cells after intravenous administration of liposome-encapsulated dichloromethylene diphosphonate. Am J Pathol. 1996 Oct;149(4):1271–1286. [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989 Feb;45(2):97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 1984;238(2):355–358. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]