Abstract
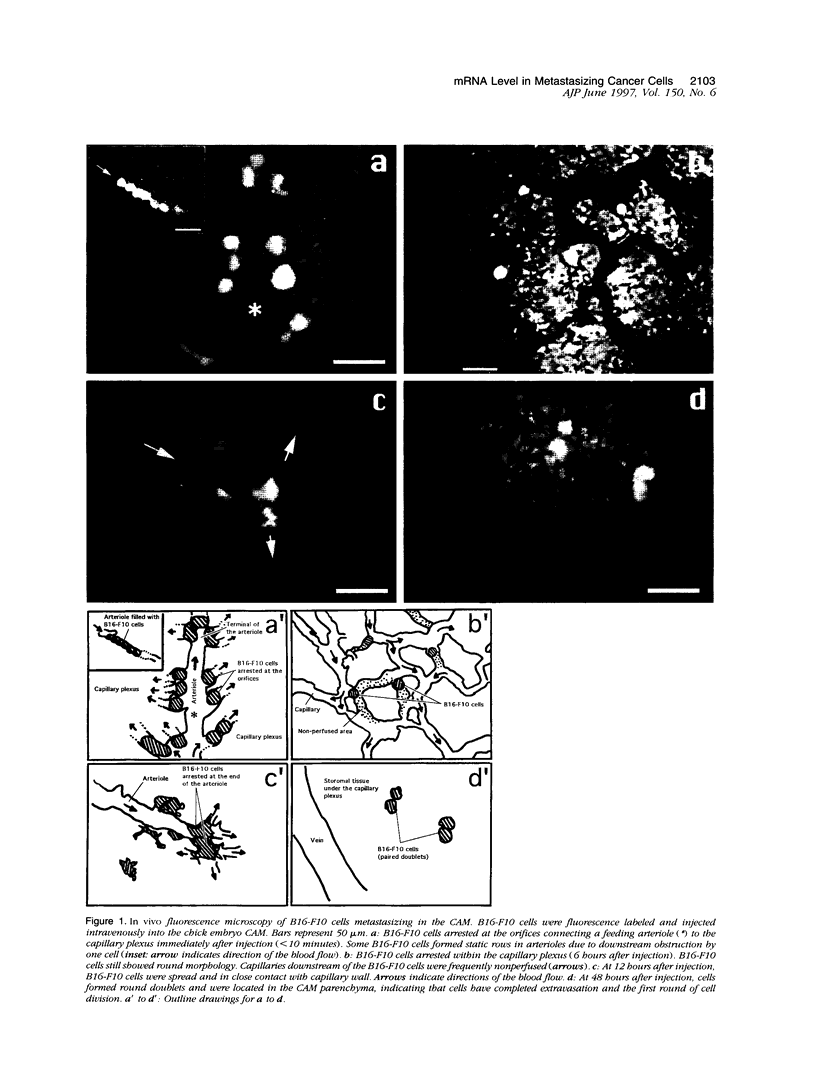
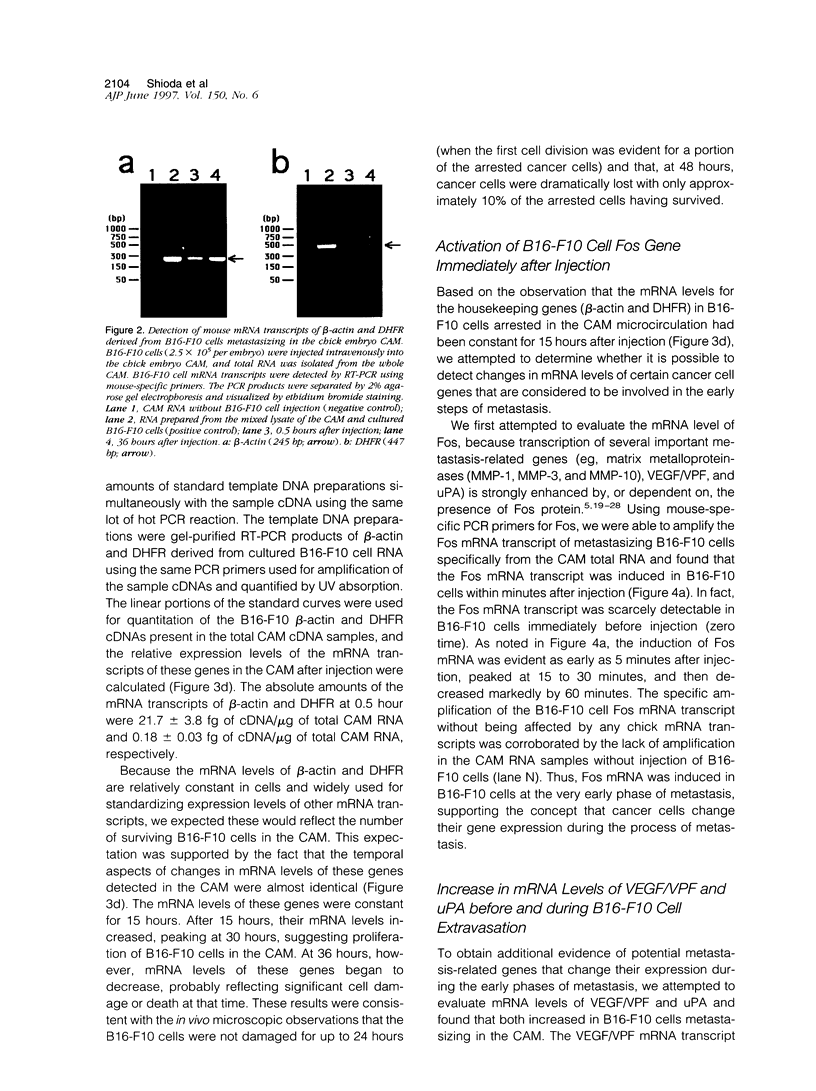
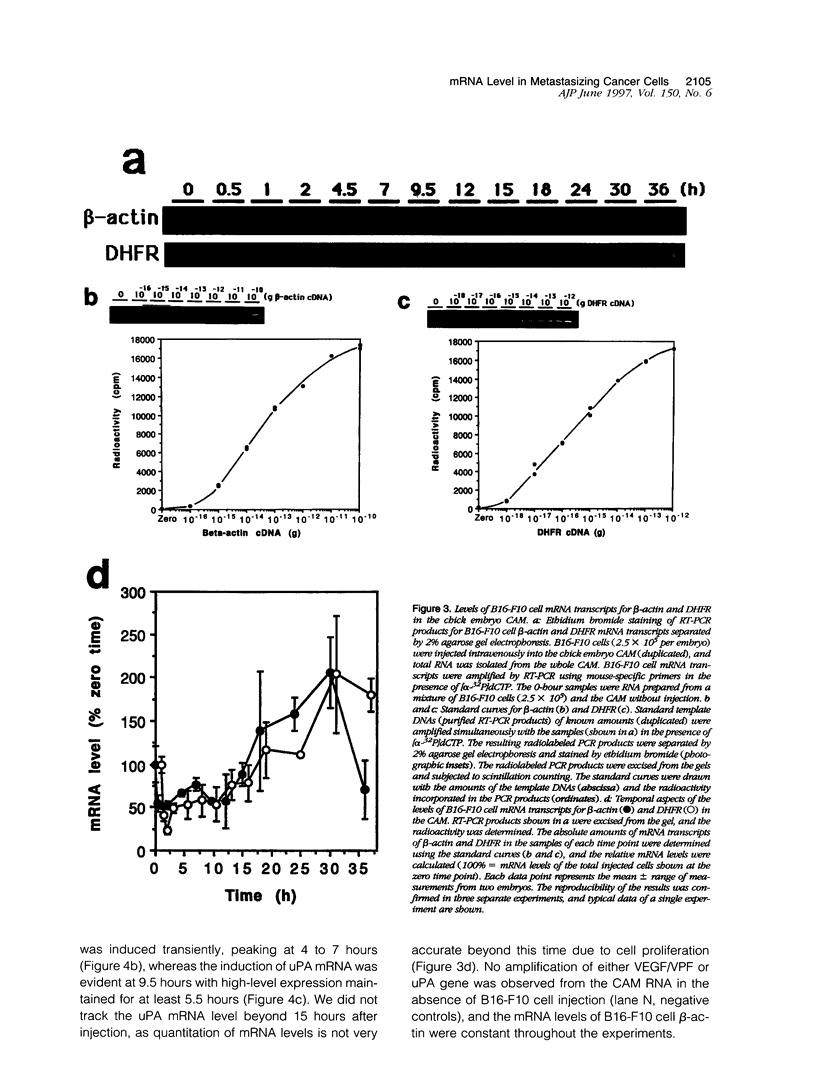
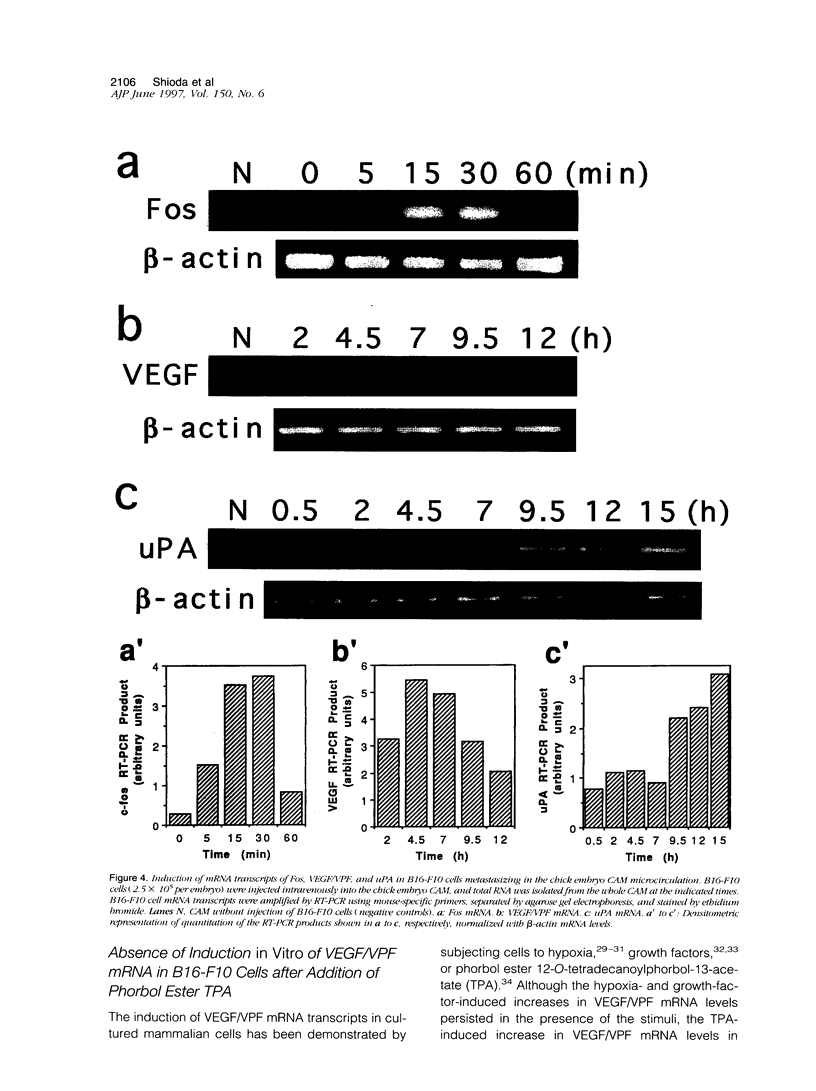
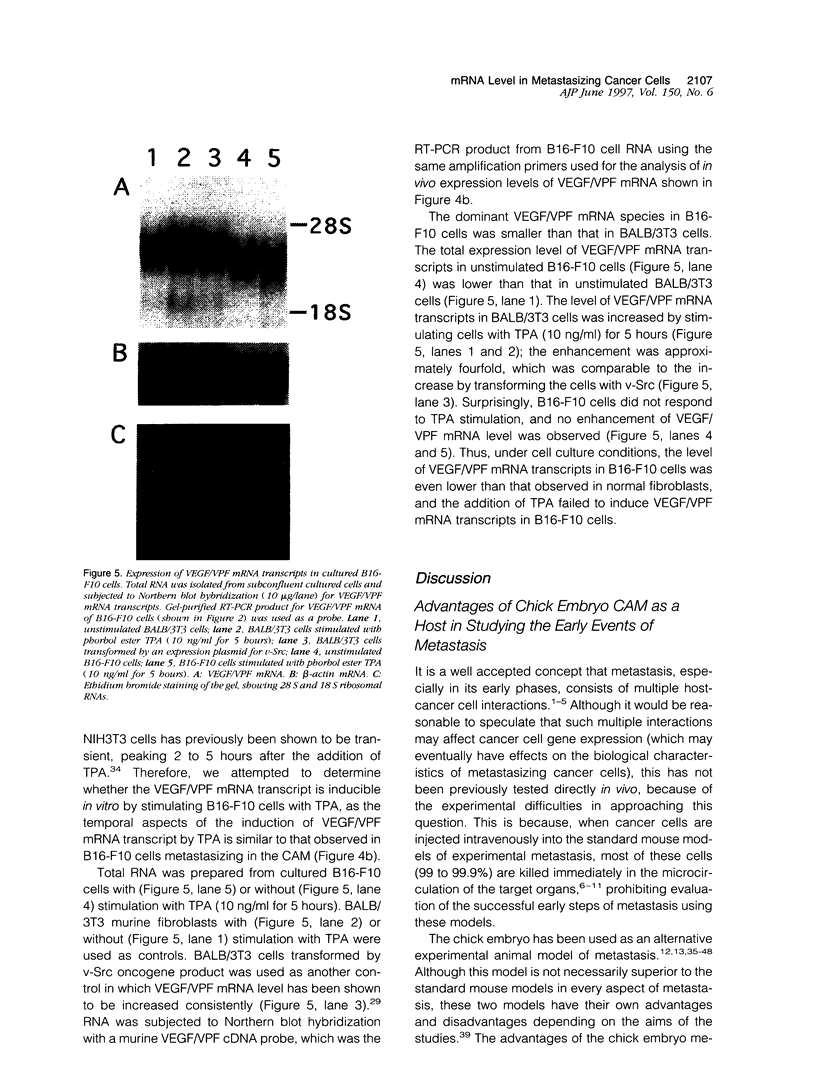
The early events of metastasis involve multiple interactions between cancer cells and the host microcirculation during cancer cell arrest, adhesion, and extravasation. These interactions may lead to changes in gene expression of the metastasizing cancer cells, although such changes have never been demonstrated directly. To test this hypothesis, B16-F10 murine melanoma cells were injected intravenously into the chick embryo chorioallantoic membrane (CAM), and mRNA levels in the metastasizing cancer cells were evaluated by species-specific reverse transcription polymerase chain reaction. Unlike standard mouse models of experimental metastasis, the CAM model showed successful extravasation of a large number of the arrested cancer cells in the CAM microcirculation without significant cancer cell death, providing a unique opportunity to keep track of mRNA levels in cancer cells during the early phases of metastasis. Using this model, we were able to demonstrate directly the temporal induction of cancer cell genes that potentially affect metastatic efficiency, namely, Fos (5 to 60 minutes after injection), vascular permeability factor (4 to 7 hours), and urokinase plasminogen activator (> 9 hours). In conclusion, using the CAM system, we have observed an alteration of gene expression in cancer cells in the early phases of metastasis, most likely as a consequence of host-cancer cell interactions. These changes may influence the metastatic behavior of cancer cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks P. C., Lin J. M., French D. L., Quigley J. P. Subtractive immunization yields monoclonal antibodies that specifically inhibit metastasis. J Cell Biol. 1993 Sep;122(6):1351–1359. doi: 10.1083/jcb.122.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P. C., Strömblad S., Sanders L. C., von Schalscha T. L., Aimes R. T., Stetler-Stevenson W. G., Quigley J. P., Cheresh D. A. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996 May 31;85(5):683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Flenniken A. M., Skup D., Williams B. R. Identification of a serum- and phorbol ester-responsive element in the murine tissue inhibitor of metalloproteinase gene. J Biol Chem. 1991 Apr 15;266(11):7199–7206. [PubMed] [Google Scholar]
- Chambers A. F., Ling V. Selection for experimental metastatic ability of heterologous tumor cells in the chick embryo after DNA-mediated transfer. Cancer Res. 1984 Sep;44(9):3970–3975. [PubMed] [Google Scholar]
- Chambers A. F., Schmidt E. E., MacDonald I. C., Morris V. L., Groom A. C. Early steps in hematogenous metastasis of B16F1 melanoma cells in chick embryos studied by high-resolution intravital videomicroscopy. J Natl Cancer Inst. 1992 May 20;84(10):797–803. doi: 10.1093/jnci/84.10.797. [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Shafir R., Ling V. A model system for studying metastasis using the embryonic chick. Cancer Res. 1982 Oct;42(10):4018–4025. [PubMed] [Google Scholar]
- Chambers A. F., Wilson S. M., Tuck A. B., Denhardt G. H., Cairncross J. G. Comparison of metastatic properties of a variety of mouse, rat, and human cells in assays in nude mice and chick embryos. In Vivo. 1990 Jul-Aug;4(4):215–219. [PubMed] [Google Scholar]
- Claffey K. P., Brown L. F., del Aguila L. F., Tognazzi K., Yeo K. T., Manseau E. J., Dvorak H. F. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996 Jan 1;56(1):172–181. [PubMed] [Google Scholar]
- Dellian M., Witwer B. P., Salehi H. A., Yuan F., Jain R. K. Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am J Pathol. 1996 Jul;149(1):59–71. [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Brown L. F., Detmar M., Dvorak A. M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995 May;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fidler I. J. Origin and biology of cancer metastasis. Cytometry. 1989 Nov;10(6):673–680. doi: 10.1002/cyto.990100602. [DOI] [PubMed] [Google Scholar]
- Frank S., Hübner G., Breier G., Longaker M. T., Greenhalgh D. G., Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995 May 26;270(21):12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- Gack S., Vallon R., Schaper J., Rüther U., Angel P. Phenotypic alterations in fos-transgenic mice correlate with changes in Fos/Jun-dependent collagenase type I expression. Regulation of mouse metalloproteinases by carcinogens, tumor promoters, cAMP, and Fos oncoprotein. J Biol Chem. 1994 Apr 8;269(14):10363–10369. [PubMed] [Google Scholar]
- Gorelik E., Wiltrout R. H., Okumura K., Habu S., Herberman R. B. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. Int J Cancer. 1982 Jul 15;30(1):107–112. doi: 10.1002/ijc.2910300118. [DOI] [PubMed] [Google Scholar]
- Grugel S., Finkenzeller G., Weindel K., Barleon B., Marmé D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995 Oct 27;270(43):25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- Hanna N. Expression of metastatic potential of tumor cells in young nude mice is correlated with low levels of natural killer cell-mediated cytotoxicity. Int J Cancer. 1980 Nov 15;26(5):675–680. doi: 10.1002/ijc.2910260521. [DOI] [PubMed] [Google Scholar]
- Hanna N., Fidler I. J. Role of natural killer cells in the destruction of circulating tumor emboli. J Natl Cancer Inst. 1980 Oct;65(4):801–809. doi: 10.1093/jnci/65.4.801. [DOI] [PubMed] [Google Scholar]
- Hart I. R., Saini A. Biology of tumour metastasis. Lancet. 1992 Jun 13;339(8807):1453–1457. doi: 10.1016/0140-6736(92)92039-i. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Law L. W., Corti A., Appella E., Blasi F. Modulation of metastatic potential by cell surface urokinase of murine melanoma cells. Cancer Res. 1988 Mar 1;48(5):1270–1278. [PubMed] [Google Scholar]
- Hu E., Mueller E., Oliviero S., Papaioannou V. E., Johnson R., Spiegelman B. M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994 Jul 1;13(13):3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Matsumoto K., White S. L., Olden K. Inhibition of experimental metastasis by castanospermine in mice: blockage of two distinct stages of tumor colonization by oligosaccharide processing inhibitors. Cancer Res. 1986 Oct;46(10):5215–5222. [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- KARNOFSKY D. A., RIDGWAY L. P., PATTERSON P. A. Tumor transplantation to the chick embryo. Ann N Y Acad Sci. 1952 Aug 8;55(2):313–329. doi: 10.1111/j.1749-6632.1952.tb26547.x. [DOI] [PubMed] [Google Scholar]
- Khokha R. Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melanoma cells in vivo by the overexpression of the tissue inhibitor of the metalloproteinases-1. J Natl Cancer Inst. 1994 Feb 16;86(4):299–304. doi: 10.1093/jnci/86.4.299. [DOI] [PubMed] [Google Scholar]
- Khokha R., Zimmer M. J., Wilson S. M., Chambers A. F. Up-regulation of TIMP-1 expression in B16-F10 melanoma cells suppresses their metastatic ability in chick embryo. Clin Exp Metastasis. 1992 Nov;10(6):365–370. doi: 10.1007/BF00133464. [DOI] [PubMed] [Google Scholar]
- Kook Y. H., Adamski J., Zelent A., Ossowski L. The effect of antisense inhibition of urokinase receptor in human squamous cell carcinoma on malignancy. EMBO J. 1994 Sep 1;13(17):3983–3991. doi: 10.1002/j.1460-2075.1994.tb06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koop S., Khokha R., Schmidt E. E., MacDonald I. C., Morris V. L., Chambers A. F., Groom A. C. Overexpression of metalloproteinase inhibitor in B16F10 cells does not affect extravasation but reduces tumor growth. Cancer Res. 1994 Sep 1;54(17):4791–4797. [PubMed] [Google Scholar]
- Koop S., MacDonald I. C., Luzzi K., Schmidt E. E., Morris V. L., Grattan M., Khokha R., Chambers A. F., Groom A. C. Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res. 1995 Jun 15;55(12):2520–2523. [PubMed] [Google Scholar]
- Koop S., Schmidt E. E., MacDonald I. C., Morris V. L., Khokha R., Grattan M., Leone J., Chambers A. F., Groom A. C. Independence of metastatic ability and extravasation: metastatic ras-transformed and control fibroblasts extravasate equally well. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11080–11084. doi: 10.1073/pnas.93.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. P., Levy N. S., Wegner S., Goldberg M. A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995 Jun 2;270(22):13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Lichtenfels R., Biddison W. E., Schulz H., Vogt A. B., Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994 Jun 24;172(2):227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Maroney A. C., Qureshi S. A., Foster D. A., Brugge J. S. Cloning and characterization of a thermolabile v-src gene for use in reversible transformation of mammalian cells. Oncogene. 1992 Jun;7(6):1207–1214. [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D., Tsiokas L., Zhou X. M., Foster D., Brugge J. S., Sukhatme V. P. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995 Jun 15;375(6532):577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- Nerlov C., Rørth P., Blasi F., Johnsen M. Essential AP-1 and PEA3 binding elements in the human urokinase enhancer display cell type-specific activity. Oncogene. 1991 Sep;6(9):1583–1592. [PubMed] [Google Scholar]
- Nguyen M., Shing Y., Folkman J. Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res. 1994 Jan;47(1):31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Antibodies to plasminogen activator inhibit human tumor metastasis. Cell. 1983 Dec;35(3 Pt 2):611–619. doi: 10.1016/0092-8674(83)90093-4. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Palade G. E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995 Jun;108(Pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Saez E., Rutberg S. E., Mueller E., Oppenheim H., Smoluk J., Yuspa S. H., Spiegelman B. M. c-fos is required for malignant progression of skin tumors. Cell. 1995 Sep 8;82(5):721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- Schiavi S. C., Wellington C. L., Shyu A. B., Chen C. Y., Greenberg M. E., Belasco J. G. Multiple elements in the c-fos protein-coding region facilitate mRNA deadenylation and decay by a mechanism coupled to translation. J Biol Chem. 1994 Feb 4;269(5):3441–3448. [PubMed] [Google Scholar]
- Schirrmacher V. Cancer metastasis: experimental approaches, theoretical concepts, and impacts for treatment strategies. Adv Cancer Res. 1985;43:1–73. doi: 10.1016/s0065-230x(08)60942-2. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Shioda T., Fenner M. H., Isselbacher K. J. Experimental animal model of hematogenous cardiac metastasis and neoplastic cardiac tamponade. J Surg Oncol. 1997 Feb;64(2):122–126. doi: 10.1002/(sici)1096-9098(199702)64:2<122::aid-jso6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Shioda T., Ohta T., Isselbacher K. J., Rhoads D. B. Differentiation-dependent expression of the Na+/glucose cotransporter (SGLT1) in LLC-PK1 cells: role of protein kinase C activation and ongoing transcription. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11919–11923. doi: 10.1073/pnas.91.25.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemeister G., Schnurr B., Mohrs K., Schächtele C., Marmé D., Martiny-Baron G. Expression of biologically active isoforms of the tumor angiogenesis factor VEGF in Escherichia coli. Biochem Biophys Res Commun. 1996 May 15;222(2):249–255. doi: 10.1006/bbrc.1996.0730. [DOI] [PubMed] [Google Scholar]
- Stavri G. T., Zachary I. C., Baskerville P. A., Martin J. F., Erusalimsky J. D. Basic fibroblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells. Synergistic interaction with hypoxia. Circulation. 1995 Jul 1;92(1):11–14. doi: 10.1161/01.cir.92.1.11. [DOI] [PubMed] [Google Scholar]
- Stein I., Neeman M., Shweiki D., Itin A., Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol. 1995 Oct;15(10):5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y., Sato H., Endo Y., Okada Y., Mai M., Sasaki T., Seiki M. Tissue inhibitor of metalloproteinase 1 is a negative regulator of the metastatic ability of a human gastric cancer cell line, KKLS, in the chick embryo. Cancer Res. 1993 Mar 15;53(6):1397–1402. [PubMed] [Google Scholar]
- Warren R. S., Yuan H., Matli M. R., Gillett N. A., Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995 Apr;95(4):1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L., Nannmark U., Johansson B. R., Bagge U. Lethal deformation of cancer cells in the microcirculation: a potential rate regulator of hematogenous metastasis. Int J Cancer. 1992 Jan 2;50(1):103–107. doi: 10.1002/ijc.2910500121. [DOI] [PubMed] [Google Scholar]
- Zhu D., Cheng C. F., Pauli B. U. Blocking of lung endothelial cell adhesion molecule-1 (Lu-ECAM-1) inhibits murine melanoma lung metastasis. J Clin Invest. 1992 Jun;89(6):1718–1724. doi: 10.1172/JCI115773. [DOI] [PMC free article] [PubMed] [Google Scholar]