Abstract
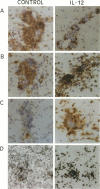
Acute, monophasic experimental allergic encephalomyelitis (EAE) in the Lewis rat shows pathological similarities to the human disease multiple sclerosis (MS). Rats that recover from EAE are essentially resistant to disease reinduction, unlike MS in which relapses are frequently associated with common bacterial and viral infections. As macrophage-derived interleukin (IL)-12 is a critical component of innate resistance to bacterial infection and appears to directly activate encephalitogenic T cells in vivo, the ability of this cytokine to reinduce paralysis in EAE was examined. Paralytic disease was exacerbated by intraperitoneal IL-12 administration and could be reinduced up to 1 week after recovery from the primary clinical episode. Concomitant with worsening of initial clinical signs and relapse was an increase in the ratio of macrophages to T cells in brain stem perivascular cuffs and the expression of inducible nitric oxide synthase in cells with both macrophage and microglial morphology. These findings suggest that IL-12 may contribute to macrophage-mediated disease exacerbation and relapse in patients with MS.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adda D. H., Beraud E., Depieds R. Evidence for suppressor cells in Lewis rats' experimental allergic encephalomyelitis. Eur J Immunol. 1977 Sep;7(9):620–623. doi: 10.1002/eji.1830070908. [DOI] [PubMed] [Google Scholar]
- Andersen O., Lygner P. E., Bergström T., Andersson M., Vahlne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. 1993 Jul;240(7):417–422. doi: 10.1007/BF00867354. [DOI] [PubMed] [Google Scholar]
- Arnon R., Teitelbaum D. On the existence of suppressor cells. Int Arch Allergy Immunol. 1993;100(1):2–7. doi: 10.1159/000236379. [DOI] [PubMed] [Google Scholar]
- Bauer J., Ruuls S. R., Huitinga I., Dijkstra C. D. The role of macrophage subpopulations in autoimmune disease of the central nervous system. Histochem J. 1996 Feb;28(2):83–97. doi: 10.1007/BF02331413. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol. 1982 Jul;129(1):303–308. [PubMed] [Google Scholar]
- Billiau A., Heremans H., Vandekerckhove F., Dijkmans R., Sobis H., Meulepas E., Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988 Mar 1;140(5):1506–1510. [PubMed] [Google Scholar]
- Brocke S., Gaur A., Piercy C., Gautam A., Gijbels K., Fathman C. G., Steinman L. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature. 1993 Oct 14;365(6447):642–644. doi: 10.1038/365642a0. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Bornstein M. B., Bloom B. R. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J Immunol. 1981 Feb;126(2):614–620. [PubMed] [Google Scholar]
- Crisi G. M., Santambrogio L., Hochwald G. M., Smith S. R., Carlino J. A., Thorbecke G. J. Staphylococcal enterotoxin B and tumor-necrosis factor-alpha-induced relapses of experimental allergic encephalomyelitis: protection by transforming growth factor-beta and interleukin-10. Eur J Immunol. 1995 Nov;25(11):3035–3040. doi: 10.1002/eji.1830251108. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Cannella B., Brosnan C. F., Raine C. S. Hypothesis: antigen-specific T cells prime central nervous system endothelium for recruitment of nonspecific inflammatory cells to effect autoimmune demyelination. J Neuroimmunol. 1991 Sep;33(3):237–244. doi: 10.1016/0165-5728(91)90111-j. [DOI] [PubMed] [Google Scholar]
- Cross A. H., Misko T. P., Lin R. F., Hickey W. F., Trotter J. L., Tilton R. G. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994 Jun;93(6):2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes R. J. Molecular alterations in the aging immune system. J Exp Med. 1995 Jul 1;182(1):1–3. doi: 10.1084/jem.182.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitinga I., van Rooijen N., de Groot C. J., Uitdehaag B. M., Dijkstra C. D. Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med. 1990 Oct 1;172(4):1025–1033. doi: 10.1084/jem.172.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issazadeh S., Ljungdahl A., Höjeberg B., Mustafa M., Olsson T. Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: dynamics of mRNA expression for interleukin-10, interleukin-12, cytolysin, tumor necrosis factor alpha and tumor necrosis factor beta. J Neuroimmunol. 1995 Sep;61(2):205–212. doi: 10.1016/0165-5728(95)00100-g. [DOI] [PubMed] [Google Scholar]
- Issazadeh S., Mustafa M., Ljungdahl A., Höjeberg B., Dagerlind A., Elde R., Olsson T. Interferon gamma, interleukin 4 and transforming growth factor beta in experimental autoimmune encephalomyelitis in Lewis rats: dynamics of cellular mRNA expression in the central nervous system and lymphoid cells. J Neurosci Res. 1995 Apr 1;40(5):579–590. doi: 10.1002/jnr.490400503. [DOI] [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991 Feb 15;146(4):1163–1168. [PubMed] [Google Scholar]
- Keith A. B. Sex difference in Lewis rats in the incidence of recurrent experimental allergic encephalomyelitis. Nature. 1978 Apr 27;272(5656):824–825. doi: 10.1038/272824a0. [DOI] [PubMed] [Google Scholar]
- Kennedy M. K., Torrance D. S., Picha K. S., Mohler K. M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992 Oct 1;149(7):2496–2505. [PubMed] [Google Scholar]
- Killen J. A., Swanborg R. H. Regulation of experimental allergic encephalomyelitis. Part 4. Further characterization of postrecovery suppressor cells. J Neuroimmunol. 1982 Oct;3(2):159–166. doi: 10.1016/0165-5728(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Kojima K., Berger T., Lassmann H., Hinze-Selch D., Zhang Y., Gehrmann J., Reske K., Wekerle H., Linington C. Experimental autoimmune panencephalitis and uveoretinitis transferred to the Lewis rat by T lymphocytes specific for the S100 beta molecule, a calcium binding protein of astroglia. J Exp Med. 1994 Sep 1;180(3):817–829. doi: 10.1084/jem.180.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H., Zimprich F., Rössler K., Vass K. Inflammation in the nervous system. Basic mechanisms and immunological concepts. Rev Neurol (Paris) 1991;147(12):763–781. [PubMed] [Google Scholar]
- Leonard J. P., Waldburger K. E., Goldman S. J. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995 Jan 1;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee I. A., Antoni F. A., Mason D. W. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med. 1989 Feb 1;169(2):431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee I. A., Mason D. W. Studies on the refractoriness to reinduction of experimental allergic encephalomyelitis in Lewis rats that have recovered from one episode of the disease. J Neuroimmunol. 1990 Apr;27(1):9–19. doi: 10.1016/0165-5728(90)90131-6. [DOI] [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., Blank S. E., Kibler R. F. Recurrent experimental allergic encephalomyelitis in the Lewis rat. J Immunol. 1974 Aug;113(2):712–715. [PubMed] [Google Scholar]
- Merrill J. E., Ignarro L. J., Sherman M. P., Melinek J., Lane T. E. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993 Aug 15;151(4):2132–2141. [PubMed] [Google Scholar]
- Merrill J. E., Kono D. H., Clayton J., Ando D. G., Hinton D. R., Hofman F. M. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic B., Ignarro L. J., Montestruque S., Smoll A., Merrill J. E. Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience. 1994 Aug;61(3):575–585. doi: 10.1016/0306-4522(94)90435-9. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Murphy S., Simmons M. L., Agullo L., Garcia A., Feinstein D. L., Galea E., Reis D. J., Minc-Golomb D., Schwartz J. P. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. 1993 Aug;16(8):323–328. doi: 10.1016/0166-2236(93)90109-y. [DOI] [PubMed] [Google Scholar]
- Okuda Y., Nakatsuji Y., Fujimura H., Esumi H., Ogura T., Yanagihara T., Sakoda S. Expression of the inducible isoform of nitric oxide synthase in the central nervous system of mice correlates with the severity of actively induced experimental allergic encephalomyelitis. J Neuroimmunol. 1995 Oct;62(1):103–112. doi: 10.1016/0165-5728(95)00114-h. [DOI] [PubMed] [Google Scholar]
- Orange J. S., Wolf S. F., Biron C. A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994 Feb 1;152(3):1253–1264. [PubMed] [Google Scholar]
- Polman C. H., Matthaei I., de Groot C. J., Koetsier J. C., Sminia T., Dijkstra C. D. Low-dose cyclosporin A induces relapsing remitting experimental allergic encephalomyelitis in the Lewis rat. J Neuroimmunol. 1988 Feb;17(3):209–216. doi: 10.1016/0165-5728(88)90069-0. [DOI] [PubMed] [Google Scholar]
- Powell M. B., Mitchell D., Lederman J., Buckmeier J., Zamvil S. S., Graham M., Ruddle N. H., Steinman L. Lymphotoxin and tumor necrosis factor-alpha production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol. 1990;2(6):539–544. doi: 10.1093/intimm/2.6.539. [DOI] [PubMed] [Google Scholar]
- Schapiro R. T., van den Noort S., Scheinberg L. The current management of multiple sclerosis. Ann N Y Acad Sci. 1984;436:425–434. doi: 10.1111/j.1749-6632.1984.tb14815.x. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D. Long-term depletion of CD8+ T cells in vivo in the rat: no observed role for CD8+ (cytotoxic/suppressor) cells in the immunoregulation of experimental allergic encephalomyelitis. Eur J Immunol. 1988 Apr;18(4):495–502. doi: 10.1002/eji.1830180402. [DOI] [PubMed] [Google Scholar]
- Segal B. M., Shevach E. M. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med. 1996 Aug 1;184(2):771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler L. A., Wink D. A., Melillo G., Cox G. W. Exogenous nitric oxide regulates IFN-gamma plus lipopolysaccharide-induced nitric oxide synthase expression in mouse macrophages. J Immunol. 1995 Jul 15;155(2):886–894. [PubMed] [Google Scholar]
- Smith T., Schmied M., Hewson A. K., Lassmann H., Cuzner M. L. Apoptosis of T cells and macrophages in the central nervous system of intact and adrenalectomized Lewis rats during experimental allergic encephalomyelitis. J Autoimmun. 1996 Apr;9(2):167–174. doi: 10.1006/jaut.1996.0020. [DOI] [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabi Z., McCombe P. A., Pender M. P. Antigen-specific down-regulation of myelin basic protein-reactive T cells during spontaneous recovery from experimental autoimmune encephalomyelitis: further evidence of apoptotic deletion of autoreactive T cells in the central nervous system. Int Immunol. 1995 Jun;7(6):967–973. doi: 10.1093/intimm/7.6.967. [DOI] [PubMed] [Google Scholar]
- Tabi Z., McCombe P. A., Pender M. P. Apoptotic elimination of V beta 8.2+ cells from the central nervous system during recovery from experimental autoimmune encephalomyelitis induced by the passive transfer of V beta 8.2+ encephalitogenic T cells. Eur J Immunol. 1994 Nov;24(11):2609–2617. doi: 10.1002/eji.1830241107. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Voorthuis J. A., Uitdehaag B. M., De Groot C. J., Goede P. H., van der Meide P. H., Dijkstra C. D. Suppression of experimental allergic encephalomyelitis by intraventricular administration of interferon-gamma in Lewis rats. Clin Exp Immunol. 1990 Aug;81(2):183–188. doi: 10.1111/j.1365-2249.1990.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldburger K. E., Hastings R. C., Schaub R. G., Goldman S. J., Leonard J. P. Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms. Am J Pathol. 1996 Feb;148(2):375–382. [PMC free article] [PubMed] [Google Scholar]
- Waxman F. J., Bergman R. K., Munoz J. J. Abrogation of resistance to the reinduction of experimental allergic encephalomyelitis by pertussigen. Cell Immunol. 1982 Sep 15;72(2):375–383. doi: 10.1016/0008-8749(82)90486-5. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Temple P. A., Kobayashi M., Young D., Dicig M., Lowe L., Dzialo R., Fitz L., Ferenz C., Hewick R. M. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991 May 1;146(9):3074–3081. [PubMed] [Google Scholar]
- Zeine R., Owens T. Loss rather than downregulation of CD4+ T cells as a mechanism for remission from experimental allergic encephalomyelitis. J Neuroimmunol. 1993 May;44(2):193–198. doi: 10.1016/0165-5728(93)90042-w. [DOI] [PubMed] [Google Scholar]