Abstract
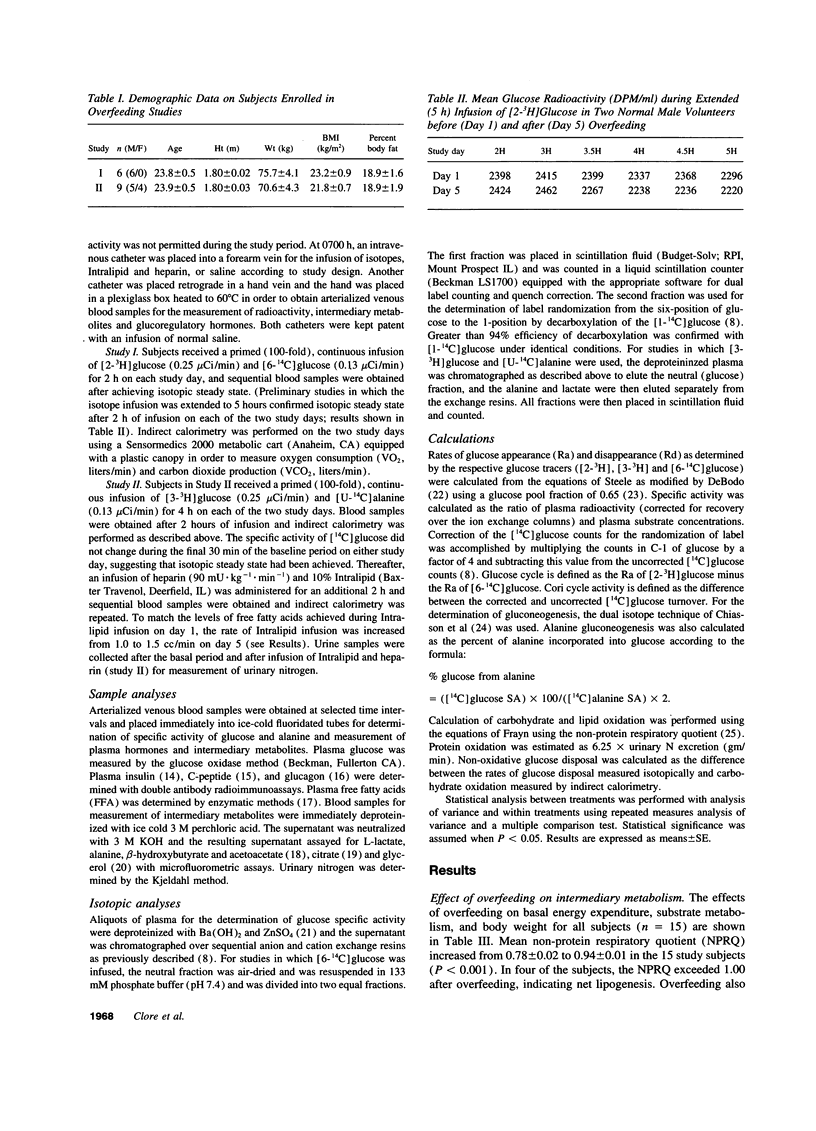
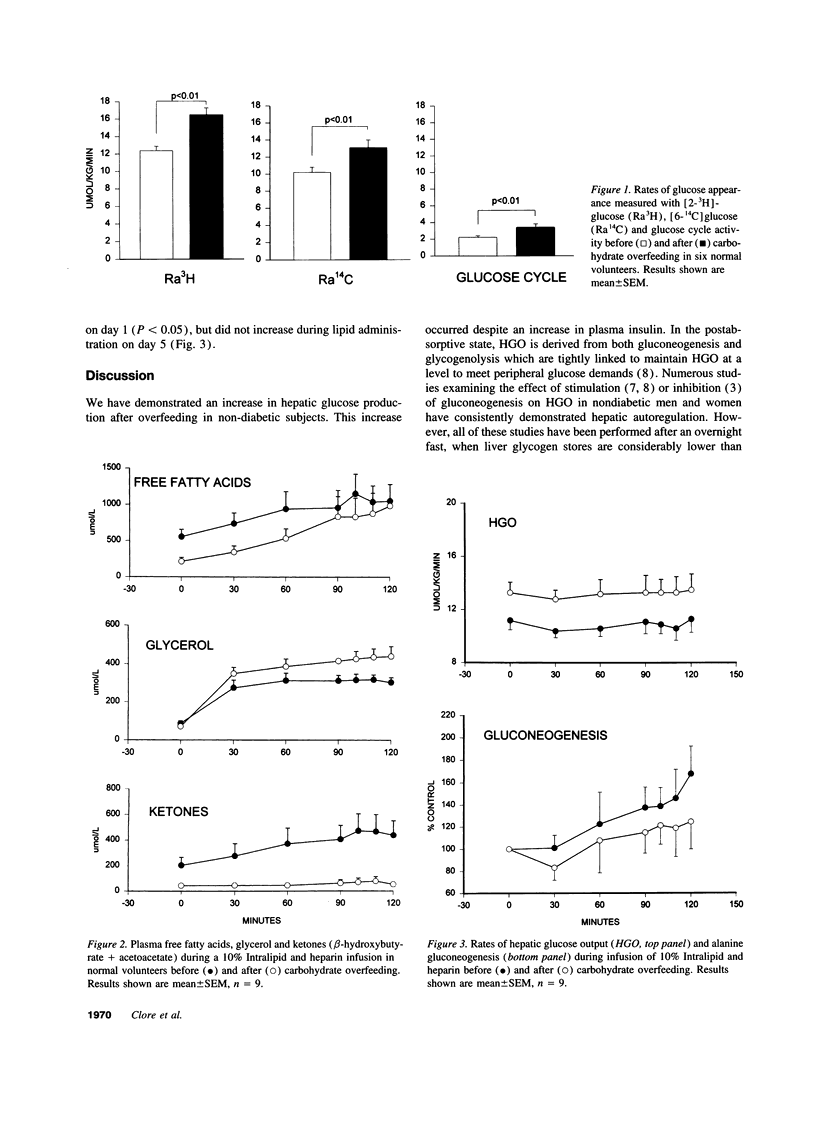
To determine the effect of increased glycogen stores on hepatic carbohydrate metabolism, 15 nondiabetic volunteers were studied before and after 4 d of progressive overfeeding. Glucose production and gluconeogenesis were assessed with [2-3H] glucose and [6-14C] glucose (Study I, n = 6) or [3-3H] glucose and [U-14C]-alanine (Study II, n = 9) and substrate oxidation was determined by indirect calorimetry. Overfeeding was associated with significant (P < 0.01) increases in plasma glucose (4.97 +/- 0.10 to 5.09 +/- 0.11 mmol/liter), insulin (18.8 +/- 1.5 to 46.6 +/- 10.0 pmol/liter) and carbohydrate oxidation (4.7 +/- 1.4 to 18.0 +/- 1.5 mumol.kg-1.min-1) and a decrease in lipid oxidation (1.2 +/- 0.2 to 0.3 +/- 0.1 mumol.kg-1.min-1). Hepatic glucose output (HGO) increased in Study I (10.2 +/- 0.5 to 13.1 +/- 0.9 mumol.kg-1.min-1, P < 0.01) and Study II (11.17 +/- 0.67 to 13.33 +/- 0.83 mumol.kg-1.min-1, P < 0.01), and gluconeogenesis decreased (57.6 +/- 6.4 to 33.4 +/- 4.9 mumol/min, P < 0.01), indicating an increase in glycogenolysis. The increase in glycogenolysis was only partly compensated by an increase in glucose cycle activity (2.2 +/- 0.2 to 3.4 +/- 0.4 mumol.kg-1.min-1, P < 0.01) and the fall in gluconeogenesis, thus resulting in increased HGO. The suppression of gluconeogenesis despite increased lactate and alanine (glycerol was decreased) was associated with decreased free fatty acid (FFA) oxidation and negligible FFA enhanced gluconeogenesis. These studies suggest that increased liver glycogen stores alone can overwhelm normal intrahepatic mechanisms regulating carbohydrate metabolism resulting in increased HGO in nondiabetic man.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson K. J., Schutz Y., Bessard T., Anantharaman K., Flatt J. P., Jéquier E. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Am J Clin Nutr. 1988 Aug;48(2):240–247. doi: 10.1093/ajcn/48.2.240. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Barzilai N., Rossetti L. Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem. 1993 Nov 25;268(33):25019–25025. [PubMed] [Google Scholar]
- Björntorp P., Sjöström L. Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism. 1978 Dec;27(12 Suppl 2):1853–1865. doi: 10.1016/s0026-0495(78)80004-3. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- Clore J. N., Blackard W. G. Suppression of gluconeogenesis after a 3-day fast does not deplete liver glycogen in patients with NIDDM. Diabetes. 1994 Feb;43(2):256–262. doi: 10.2337/diab.43.2.256. [DOI] [PubMed] [Google Scholar]
- Clore J. N., Glickman P. S., Nestler J. E., Blackard W. G. In vivo evidence for hepatic autoregulation during FFA-stimulated gluconeogenesis in normal humans. Am J Physiol. 1991 Oct;261(4 Pt 1):E425–E429. doi: 10.1152/ajpendo.1991.261.4.E425. [DOI] [PubMed] [Google Scholar]
- Clore J. N., Post E. P., Bailey D. J., Nestler J. E., Blackard W. G. Evidence for increased liver glycogen in patients with noninsulin-dependent diabetes mellitus after a 3-day fast. J Clin Endocrinol Metab. 1992 Mar;74(3):660–666. doi: 10.1210/jcem.74.3.1740502. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- David M., Petit W. A., Laughlin M. R., Shulman R. G., King J. E., Barrett E. J. Simultaneous synthesis and degradation of rat liver glycogen. An in vivo nuclear magnetic resonance spectroscopic study. J Clin Invest. 1990 Aug;86(2):612–617. doi: 10.1172/JCI114752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnin J. V., Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974 Jul;32(1):77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- Efendic S., Karlander S., Vranic M. Mild type II diabetes markedly increases glucose cycling in the postabsorptive state and during glucose infusion irrespective of obesity. J Clin Invest. 1988 Jun;81(6):1953–1961. doi: 10.1172/JCI113543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Garfield S. A., Cardell R. R., Jr Hepatic glucose-6-phosphatase activities and correlated ultrastructural alterations in hepatocytes of diabetic rats. Diabetes. 1979 Jul;28(7):664–679. doi: 10.2337/diab.28.7.664. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B. Mammalian glucokinase and its gene. Biochem J. 1993 Jul 1;293(Pt 1):1–13. doi: 10.1042/bj2930001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoor F., Peters E. J., Wolfe R. R. The relationship between gluconeogenic substrate supply and glucose production in humans. Am J Physiol. 1990 Feb;258(2 Pt 1):E288–E296. doi: 10.1152/ajpendo.1990.258.2.E288. [DOI] [PubMed] [Google Scholar]
- Jeng C. Y., Sheu W. H., Fuh M. M., Chen Y. D., Reaven G. M. Relationship between hepatic glucose production and fasting plasma glucose concentration in patients with NIDDM. Diabetes. 1994 Dec;43(12):1440–1444. doi: 10.2337/diab.43.12.1440. [DOI] [PubMed] [Google Scholar]
- Jenssen T., Nurjhan N., Consoli A., Gerich J. E. Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. J Clin Invest. 1990 Aug;86(2):489–497. doi: 10.1172/JCI114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher J. K. Gluconeogenesis from labeled carbon: estimating isotope dilution. Am J Physiol. 1986 Mar;250(3 Pt 1):E296–E305. doi: 10.1152/ajpendo.1986.250.3.E296. [DOI] [PubMed] [Google Scholar]
- Kubota M., Virkamäki A., Yki-Järvinen H. Ethanol stimulates glycogenolysis in livers from fed rats. Proc Soc Exp Biol Med. 1992 Oct;201(1):114–118. doi: 10.3181/00379727-201-43488. [DOI] [PubMed] [Google Scholar]
- Kumar M. S., Schumacher O. P., Deodhar S. D. Measurement of serum C-peptide immunoreactivity by radioimmunoassay in insulin-dependent diabetics. Am J Clin Pathol. 1980 Jul;74(1):78–82. doi: 10.1093/ajcp/74.1.78. [DOI] [PubMed] [Google Scholar]
- Kushner R. F. Bioelectrical impedance analysis: a review of principles and applications. J Am Coll Nutr. 1992 Apr;11(2):199–209. [PubMed] [Google Scholar]
- Landau B. R. Measuring glucose and fructose-6-phosphate cycling in liver in vivo. Metabolism. 1993 Apr;42(4):457–462. doi: 10.1016/0026-0495(93)90103-u. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Jucker B., Cline G. W., Shulman R. G., Shulman G. I. Liver glycogen turnover in fed and fasted humans. Am J Physiol. 1994 May;266(5 Pt 1):E796–E803. doi: 10.1152/ajpendo.1994.266.5.E796. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan R. J. A simple, rapid method for the determination of glucose, lactate, pyruvate, alanine, 3-hydroxybutyrate and acetoacetate on a single 20-mul blood sample. Clin Chim Acta. 1982 Jul 1;122(2):231–240. doi: 10.1016/0009-8981(82)90282-0. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Mott D. M., Lillioja S., Bogardus C. Overnutrition induced decrease in insulin action for glucose storage: in vivo and in vitro in man. Metabolism. 1986 Feb;35(2):160–165. doi: 10.1016/0026-0495(86)90118-6. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver glycogen in man--the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest. 1973 Dec;32(4):325–330. doi: 10.3109/00365517309084355. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., el-Maghrabi M. R., Claus T. H. Fructose-2,6-bisphosphate in control of hepatic gluconeogenesis. From metabolites to molecular genetics. Diabetes Care. 1990 Jun;13(6):582–599. doi: 10.2337/diacare.13.6.582. [DOI] [PubMed] [Google Scholar]
- Puhakainen I., Koivisto V. A., Yki-Järvinen H. No reduction in total hepatic glucose output by inhibition of gluconeogenesis with ethanol in NIDDM patients. Diabetes. 1991 Oct;40(10):1319–1327. doi: 10.2337/diab.40.10.1319. [DOI] [PubMed] [Google Scholar]
- Puhakainen I., Yki-Järvinen H. Inhibition of lipolysis decreases lipid oxidation and gluconeogenesis from lactate but not fasting hyperglycemia or total hepatic glucose production in NIDDM. Diabetes. 1993 Dec;42(12):1694–1699. doi: 10.2337/diab.42.12.1694. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Magnusson I., Katz L. D., Shulman R. G., Shulman G. I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991 Oct 25;254(5031):573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- Toftegaard Nielsen T. A method for enzymatic determination of citrate in serum and urine. Scand J Clin Lab Invest. 1976 Oct;36(6):513–519. [PubMed] [Google Scholar]
- Welle S. L., Seaton T. B., Campbell R. G. Some metabolic effects of overeating in man. Am J Clin Nutr. 1986 Dec;44(6):718–724. doi: 10.1093/ajcn/44.6.718. [DOI] [PubMed] [Google Scholar]


