Abstract
A new monoclonal antibody (MAb), CNA.42, was generated using the CEM T-cell line. It recognizes a 120-kd formalin-resistant glycosylated antigen that is mainly expressed by follicular dendritic reticulum cells (FDRCs). This antigen is also expressed by a few mononuclear cells in the paracortical area of reactive lymph nodes and by some cortical thymocytes. Two hundred and eighty-nine cases of hematopoietic tumors of various types were tested with this antibody. They showed either intact FDRC networks or FDRC networks dispersed among malignant cells. In follicular lymphomas, the follicular pattern was highlighted by CNA.42 MAb. Expanded FDRC networks were found in angioimmunoblastic T-cell lymphomas. Neoplastic cells were positive in 43.6% (24/55) of T-cell and 4.6% (6/129) of B-cell lymphomas. The highest percentage of cases with positive neoplastic cells was found in anaplastic large-cell lymphomas (62.5%; 15/24). In Hodgkin's disease, FDRC networks, sometimes encasing Hodgkin and Reed-Sternberg (HRS) cells, were found. HRS cells were also stained by this antibody in 23 (21.9%) of the 105 cases examined. A variety of normal nonlymphoid tissues and nonhematopoietic tumors, such as some neurogenic tumors, carcinoma, and occasional sarcomas, were found to be positive. Analysis of the reactivity of CNA.42 antibody with FDRCs of lymphoid tissue from different animal species showed similar reactivity to that observed in humans, suggesting widespread evolutionary conservation of the antigen recognized by this antibody. In daily diagnostic practice, CNA.42 MAb seems to be a suitable FDRC marker and possibly has an auxiliary role in recognizing T-cell lymphomas.
Full text
PDF


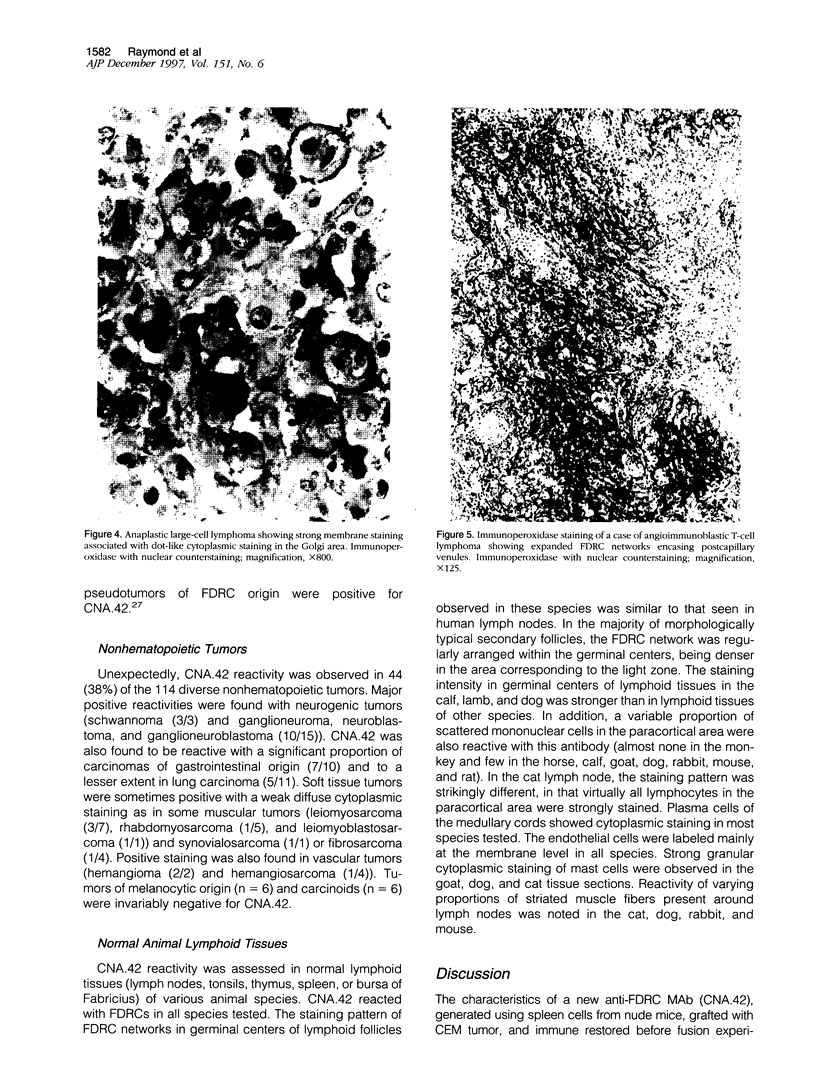
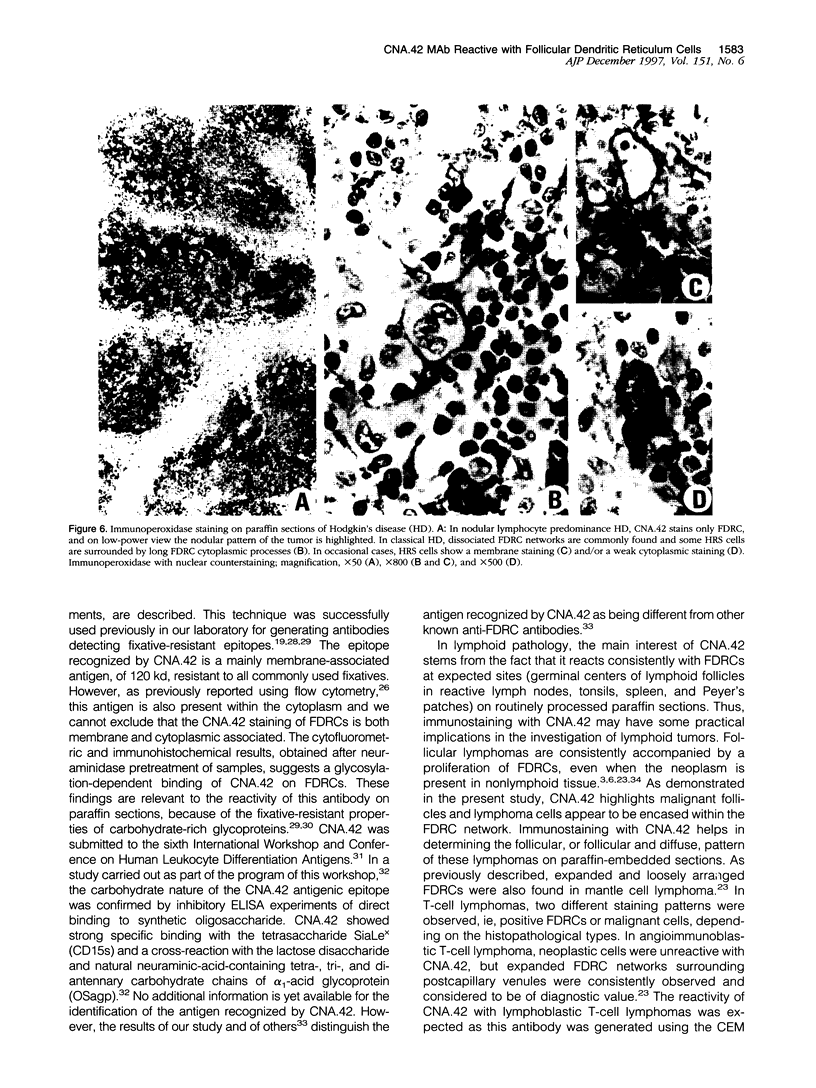


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al Saati T., Blancher A., Calvas P., Neulat-Duga I., Delsol G. Production of monoclonal antibodies using spleen cells from nude mice bearing human tumors. Ann Pathol. 1987;7(1):1–8. [PubMed] [Google Scholar]
- Alavaikko M. J., Blanco G., Aine R., Lehtinen T., Fellbaum C., Taskinen P. J., Sarpola A., Hansmann M. L. Follicular dendritic cells have prognostic relevance in Hodgkin's disease. Am J Clin Pathol. 1994 Jun;101(6):761–767. doi: 10.1093/ajcp/101.6.761. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Weber K., Droste R., Osborn M. Desmin is a specific marker for rhabdomyosarcomas of human and rat origin. Am J Pathol. 1985 Jan;118(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- Ancelin E., Delsol G., Familiades J., Mason D. Y., Kuhlein E., Al Saati T., Laurent G., Huguet-Rigal F. In situ immunologic characterization of follicular lymphomas. Hematol Oncol. 1984 Jul-Sep;2(3):221–237. doi: 10.1002/hon.2900020302. [DOI] [PubMed] [Google Scholar]
- Battifora H. The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest. 1986 Aug;55(2):244–248. [PubMed] [Google Scholar]
- Bollum F. J., Augl C., Chang L. M. Monoclonal antibodies to human terminal transferase. J Biol Chem. 1984 May 10;259(9):5848–5850. [PubMed] [Google Scholar]
- Caux C., Liu Y. J., Banchereau J. Recent advances in the study of dendritic cells and follicular dendritic cells. Immunol Today. 1995 Jan;16(1):2–4. doi: 10.1016/0167-5699(95)80061-1. [DOI] [PubMed] [Google Scholar]
- Chittal S. M., Caverivière P., Schwarting R., Gerdes J., Al Saati T., Rigal-Huguet F., Stein H., Delsol G. Monoclonal antibodies in the diagnosis of Hodgkin's disease. The search for a rational panel. Am J Surg Pathol. 1988 Jan;12(1):9–21. doi: 10.1097/00000478-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Delsol G., Al Saati T., Gatter K. C., Gerdes J., Schwarting R., Caveriviere P., Rigal-Huguet F., Robert A., Stein H., Mason D. Y. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol. 1988 Jan;130(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Delsol G., Blancher A., al Saati T., Ralfkiaer E., Lauritzen A., Bruigères L., Brousset P., Rigal-Huguet F., Mazerolles C., Robert A. Antibody BNH9 detects red blood cell-related antigens on anaplastic large cell (CD30+) lymphomas. Br J Cancer. 1991 Aug;64(2):321–326. doi: 10.1038/bjc.1991.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
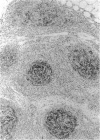
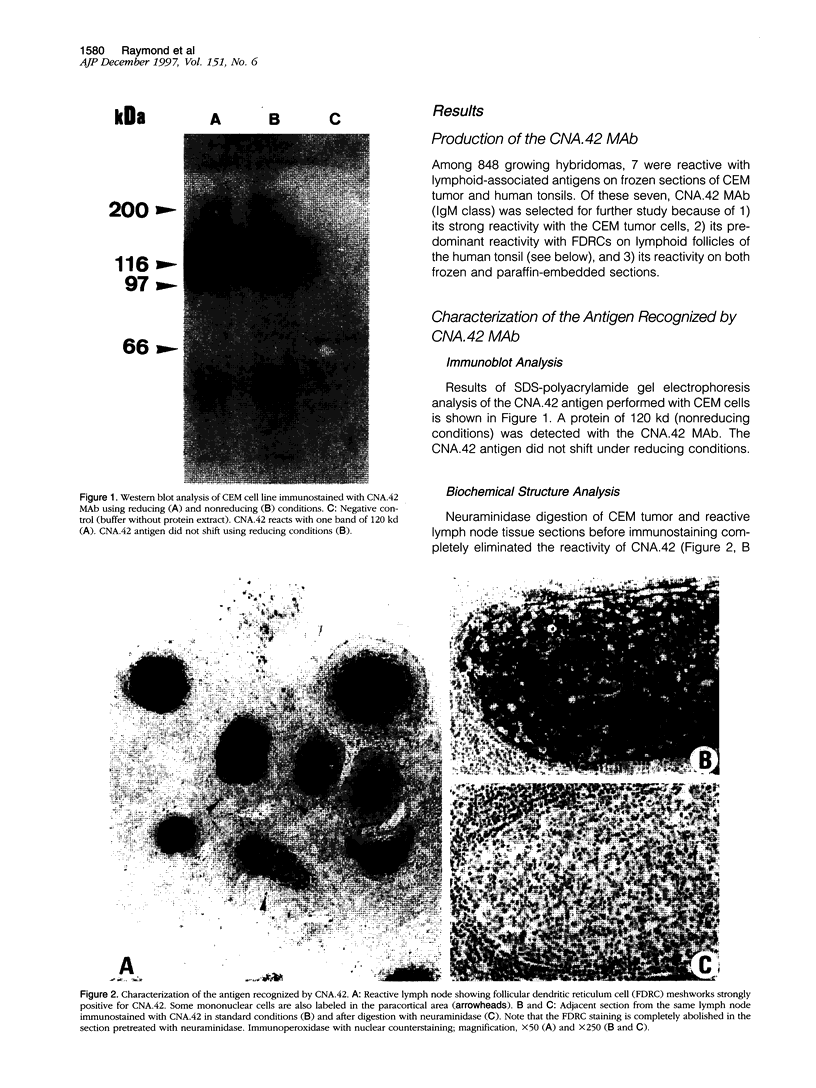
- Delsol G., Meggetto F., Brousset P., Cohen-Knafo E., al Saati T., Rochaix P., Gorguet B., Rubin B., Voigt J. J., Chittal S. Relation of follicular dendritic reticulum cells to Reed-Sternberg cells of Hodgkin's disease with emphasis on the expression of CD21 antigen. Am J Pathol. 1993 Jun;142(6):1729–1738. [PMC free article] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Falini B., Flenghi L., Fagioli M., Stein H., Schwarting R., Riccardi C., Manocchio I., Pileri S., Pelicci P. G., Lanfrancone L. Evolutionary conservation in various mammalian species of the human proliferation-associated epitope recognized by the Ki-67 monoclonal antibody. J Histochem Cytochem. 1989 Oct;37(10):1471–1478. doi: 10.1177/37.10.2476477. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Stein H., Mason D. Y., Ziegler A. Human dendritic reticulum cells of lymphoid follicles: their antigenic profile and their identification as multinucleated giant cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;42(2):161–172. doi: 10.1007/BF02890379. [DOI] [PubMed] [Google Scholar]
- Guettier C., Gatter K. C., Heryet A., Mason D. Y. Dendritic reticulum cells in reactive lymph nodes and tonsils: an immunohistological study. Histopathology. 1986 Jan;10(1):15–24. doi: 10.1111/j.1365-2559.1986.tb02457.x. [DOI] [PubMed] [Google Scholar]
- Heinen E., Bosseloir A. Follicular dendritic cells: whose children? Immunol Today. 1994 May;15(5):201–204. doi: 10.1016/0167-5699(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Heinen E. Les cellules dendritiques folliculaires: phénotype, origine et fonctions. Pathol Biol (Paris) 1995 Dec;43(10):848–857. [PubMed] [Google Scholar]
- Imal Y., Yamakawa M. Morphology, function and pathology of follicular dendritic cells. Pathol Int. 1996 Nov;46(11):807–833. doi: 10.1111/j.1440-1827.1996.tb03555.x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
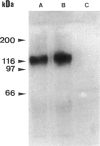
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamant L., Meggetto F., al Saati T., Brugières L., de Paillerets B. B., Dastugue N., Bernheim A., Rubie H., Terrier-Lacombe M. J., Robert A. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin's disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood. 1996 Jan 1;87(1):284–291. [PubMed] [Google Scholar]
- Lauweryns J. M., Van Ranst L. Leu-7 immunoreactivity in human, monkey, and pig bronchopulmonary neuroepithelial bodies and neuroendocrine cells. J Histochem Cytochem. 1987 Jun;35(6):687–691. doi: 10.1177/35.6.3106468. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., van Ranst L., Lloyd R. V., O'Connor D. T. Chromogranin in bronchopulmonary neuroendocrine cells. Immunocytochemical detection in human, monkey, and pig respiratory mucosa. J Histochem Cytochem. 1987 Jan;35(1):113–118. doi: 10.1177/35.1.3098831. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Arpin C. Germinal center development. Immunol Rev. 1997 Apr;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Parwaresch M. R., Radzun H. J., Hansmann M. L., Peters K. P. Monoclonal antibody Ki-M4 specifically recognizes human dendritic reticulum cells (follicular dendritic cells) and their possible precursor in blood. Blood. 1983 Sep;62(3):585–590. [PubMed] [Google Scholar]
- Peters J. P., Rademakers L. H., Roelofs J. M., de Jong D., van Unnik J. A. Distribution of dendritic reticulum cells in follicular lymphoma and reactive hyperplasia. Light microscopic identification and general morphology. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;46(3):215–228. doi: 10.1007/BF02890311. [DOI] [PubMed] [Google Scholar]
- Petrasch S., Perez-Alvarez C., Schmitz J., Kosco M., Brittinger G. Antigenic phenotyping of human follicular dendritic cells isolated from nonmalignant and malignant lymphatic tissue. Eur J Immunol. 1990 May;20(5):1013–1018. doi: 10.1002/eji.1830200510. [DOI] [PubMed] [Google Scholar]
- Pulford K., Lamant L., Morris S. W., Butler L. H., Wood K. M., Stroud D., Delsol G., Mason D. Y. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood. 1997 Feb 15;89(4):1394–1404. [PubMed] [Google Scholar]
- Rademakers L. H., Peters J. P., van Unnik J. A. Histiocytic and dendritic reticulum cells in follicular structures of follicular lymphoma and reactive hyperplasia. A quantitative electron microscopical analysis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(1):85–98. doi: 10.1007/BF02890162. [DOI] [PubMed] [Google Scholar]
- Schriever F., Freeman G., Nadler L. M. Follicular dendritic cells contain a unique gene repertoire demonstrated by single-cell polymerase chain reaction. Blood. 1991 Feb 15;77(4):787–791. [PubMed] [Google Scholar]
- Selves J., Meggetto F., Brousset P., Voigt J. J., Pradère B., Grasset D., Icart J., Mariamé B., Knecht H., Delsol G. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein-Barr virus. Am J Surg Pathol. 1996 Jun;20(6):747–753. doi: 10.1097/00000478-199606000-00013. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Stross W. P., Warnke R. A., Flavell D. J., Flavell S. U., Simmons D., Gatter K. C., Mason D. Y. Molecule detected in formalin fixed tissue by antibodies MT1, DF-T1, and L60 (Leu-22) corresponds to CD43 antigen. J Clin Pathol. 1989 Sep;42(9):953–961. doi: 10.1136/jcp.42.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al Saati T., Caspar S., Brousset P., Chittal S., Caverivière P., Hounieu H., Dastugue N., Idoipe J. B., Icart J., Mazerolles C. Production of anti-B monoclonal antibodies (DBB.42, DBA.44, DNA.7, and DND.53) reactive on paraffin-embedded tissues with a new B-lymphoma cell line grafted into athymic nude mice. Blood. 1989 Nov 15;74(7):2476–2485. [PubMed] [Google Scholar]
- al Saati T., Clamens S., Cohen-Knafo E., Faye J. C., Prats H., Coindre J. M., Wafflart J., Caverivière P., Bayard F., Delsol G. Production of monoclonal antibodies to human estrogen-receptor protein (ER) using recombinant ER (RER). Int J Cancer. 1993 Oct 21;55(4):651–654. doi: 10.1002/ijc.2910550423. [DOI] [PubMed] [Google Scholar]
- al Saati T., Tkaczuk J., Krissansen G., Print C., Pileri S., Ralfkiaer E., Grogan T. M., Meggetto F., Delsol G. A novel antigen detected by the CBF.78 antibody further distinguishes anaplastic large cell lymphoma from Hodgkin's disease. Blood. 1995 Oct 1;86(7):2741–2746. [PubMed] [Google Scholar]