Abstract
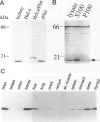
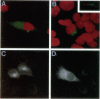
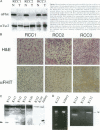
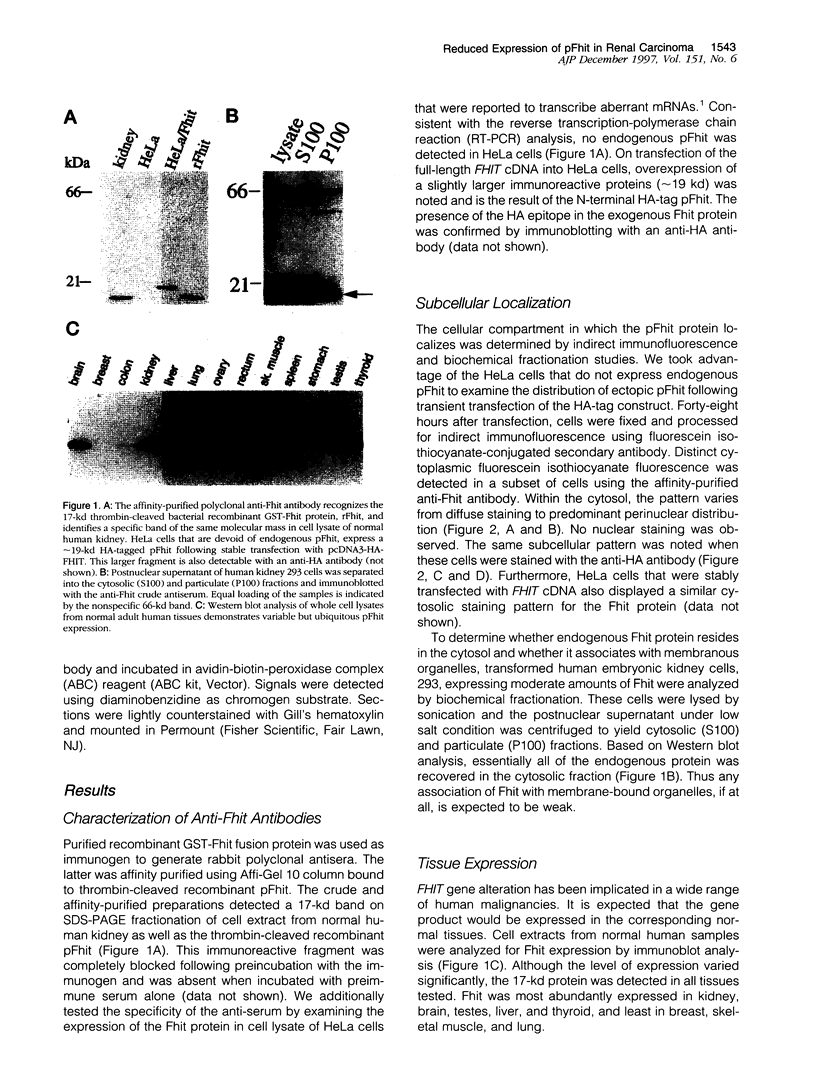
Loss of heterozygosity and homozygous deletion of the 3p14.2 region in human cancers implies the existence of a tumor suppressor gene. One such candidate is the fragile histidine triad (FHIT) gene. To investigate the role of FHIT gene product in tumorigenesis, we generated specific polyclonal antibodies to the human protein and studied its expression in normal and tumor tissues. Immunoblot analysis revealed highly variable expression of pFhit in normal adult human tissues. The highest steady-state level of pFhit was found in kidney and brain, whereas breast, intestine, and skeletal muscle expressed only trace amounts. Within the kidney, the pattern of pFhit immunoreactivity was confined to the tubular epithelium and absent in the glomeruli. Immunofluorescence analysis and biochemical fractionation have sublocalized pFhit to the cytosolic compartment. Compared with normal kidney, pFhit was found to be down-regulated in a subset of primary renal cell carcinoma. Two of 12 renal cell carcinoma cell lines that are known not to contain VHL mutations showed complete loss of pFhit expression. This is supported by the appearance of aberrant reverse transcription-polymerase chain reaction products and loss of the normal-size fragment. Our results are consistent with a potential role of pFhit loss or dysfunction in human renal cell carcinoma independent of VHL involvement.
Full text
PDF

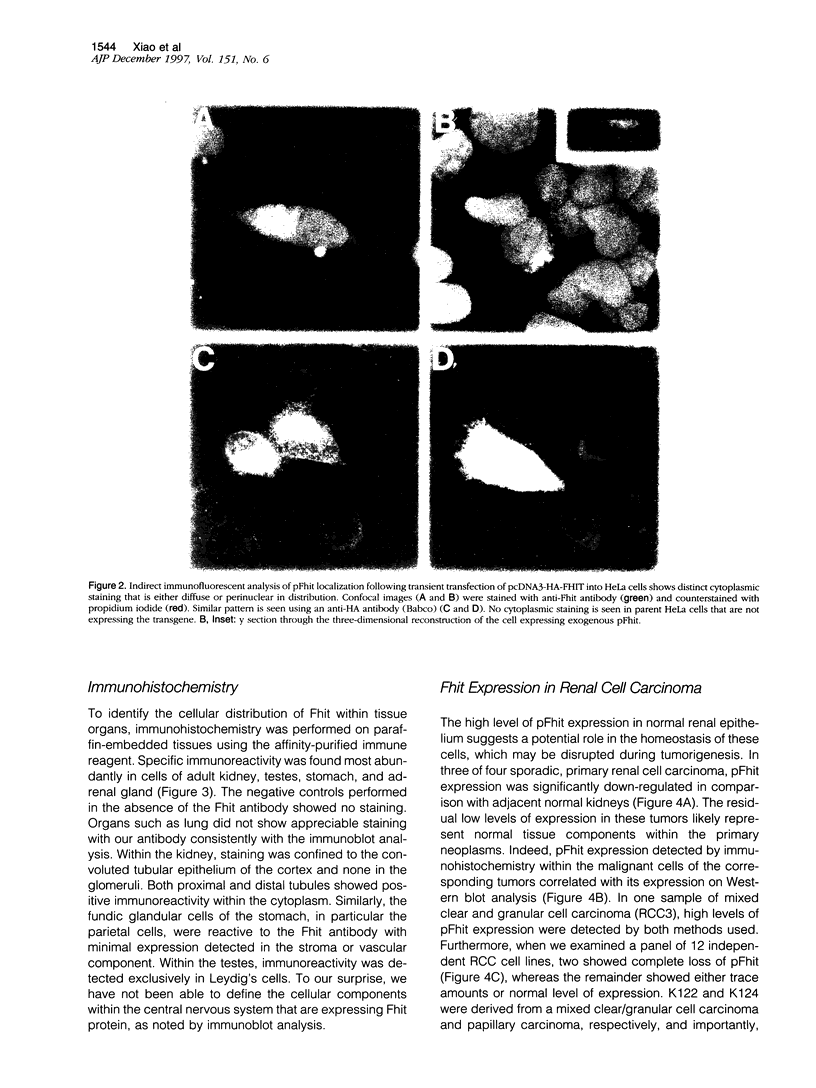

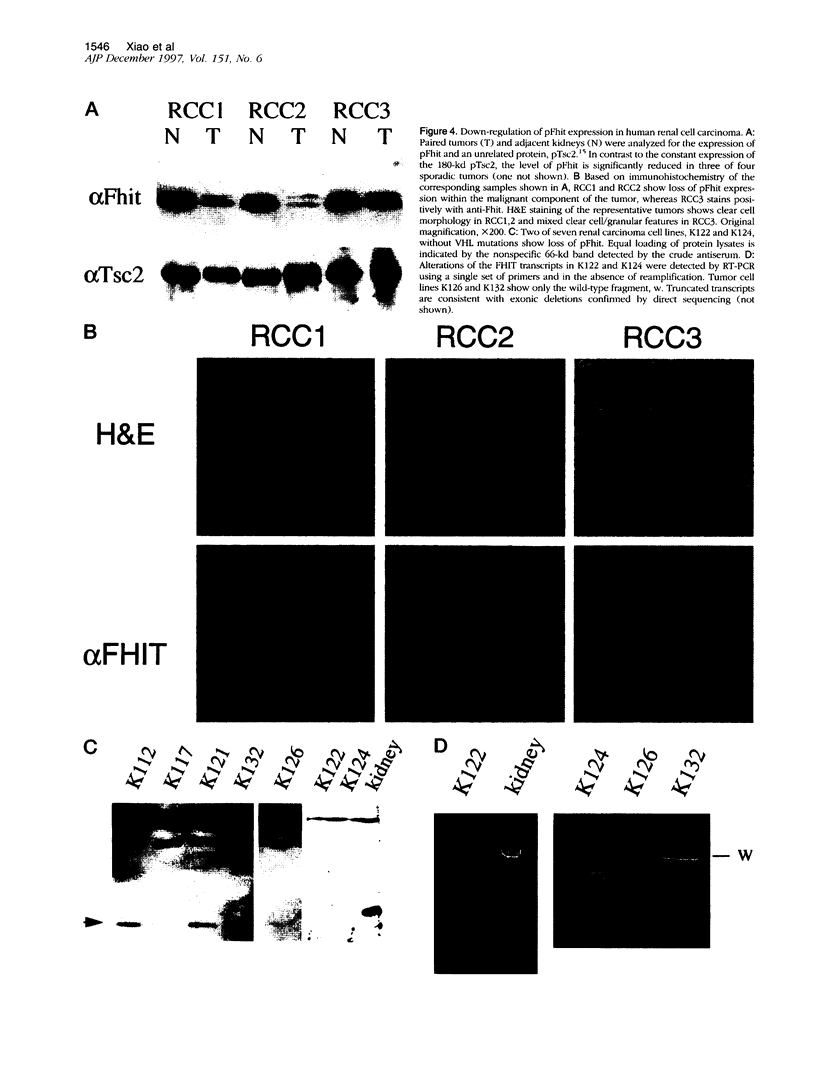

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes L. D., Garrison P. N., Siprashvili Z., Guranowski A., Robinson A. K., Ingram S. W., Croce C. M., Ohta M., Huebner K. Fhit, a putative tumor suppressor in humans, is a dinucleoside 5',5"'-P1,P3-triphosphate hydrolase. Biochemistry. 1996 Sep 10;35(36):11529–11535. doi: 10.1021/bi961415t. [DOI] [PubMed] [Google Scholar]
- Druck T., Hadaczek P., Fu T. B., Ohta M., Siprashvili Z., Baffa R., Negrini M., Kastury K., Veronese M. L., Rosen D. Structure and expression of the human FHIT gene in normal and tumor cells. Cancer Res. 1997 Feb 1;57(3):504–512. [PubMed] [Google Scholar]
- Kovacs G., Kung H. F. Nonhomologous chromatid exchange in hereditary and sporadic renal cell carcinomas. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):194–198. doi: 10.1073/pnas.88.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Fan Y. H., Lotan R., Hong W. K. Frequent abnormalities of FHIT, a candidate tumor suppressor gene, in head and neck cancer cell lines. Cancer Res. 1996 Nov 15;56(22):5128–5131. [PubMed] [Google Scholar]
- Negrini M., Monaco C., Vorechovsky I., Ohta M., Druck T., Baffa R., Huebner K., Croce C. M. The FHIT gene at 3p14.2 is abnormal in breast carcinomas. Cancer Res. 1996 Jul 15;56(14):3173–3179. [PubMed] [Google Scholar]
- Ohta M., Inoue H., Cotticelli M. G., Kastury K., Baffa R., Palazzo J., Siprashvili Z., Mori M., McCue P., Druck T. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996 Feb 23;84(4):587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- Sidhar S. K., Clark J., Gill S., Hamoudi R., Crew A. J., Gwilliam R., Ross M., Linehan W. M., Birdsall S., Shipley J. The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses a novel gene PRCC to the TFE3 transcription factor gene. Hum Mol Genet. 1996 Sep;5(9):1333–1338. doi: 10.1093/hmg/5.9.1333. [DOI] [PubMed] [Google Scholar]
- Sozzi G., Alder H., Tornielli S., Corletto V., Baffa R., Veronese M. L., Negrini M., Pilotti S., Pierotti M. A., Huebner K. Aberrant FHIT transcripts in Merkel cell carcinoma. Cancer Res. 1996 Jun 1;56(11):2472–2474. [PubMed] [Google Scholar]
- Sozzi G., Veronese M. L., Negrini M., Baffa R., Cotticelli M. G., Inoue H., Tornielli S., Pilotti S., De Gregorio L., Pastorino U. The FHIT gene 3p14.2 is abnormal in lung cancer. Cell. 1996 Apr 5;85(1):17–26. doi: 10.1016/s0092-8674(00)81078-8. [DOI] [PubMed] [Google Scholar]
- Virgilio L., Shuster M., Gollin S. M., Veronese M. L., Ohta M., Huebner K., Croce C. M. FHIT gene alterations in head and neck squamous cell carcinomas. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9770–9775. doi: 10.1073/pnas.93.18.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienecke R., Maize J. C., Jr, Shoarinejad F., Vass W. C., Reed J., Bonifacino J. S., Resau J. H., de Gunzburg J., Yeung R. S., DeClue J. E. Co-localization of the TSC2 product tuberin with its target Rap1 in the Golgi apparatus. Oncogene. 1996 Sep 5;13(5):913–923. [PubMed] [Google Scholar]
- Xiao G. H., Shoarinejad F., Jin F., Golemis E. A., Yeung R. S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J Biol Chem. 1997 Mar 7;272(10):6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Katsetos C. D., Klein-Szanto A. Subependymal astrocytic hamartomas in the Eker rat model of tuberous sclerosis. Am J Pathol. 1997 Nov;151(5):1477–1486. [PMC free article] [PubMed] [Google Scholar]
- van den Berg A., Buys C. H. Involvement of multiple loci on chromosome 3 in renal cell cancer development. Genes Chromosomes Cancer. 1997 Jun;19(2):59–76. doi: 10.1002/(sici)1098-2264(199706)19:2<59::aid-gcc1>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]