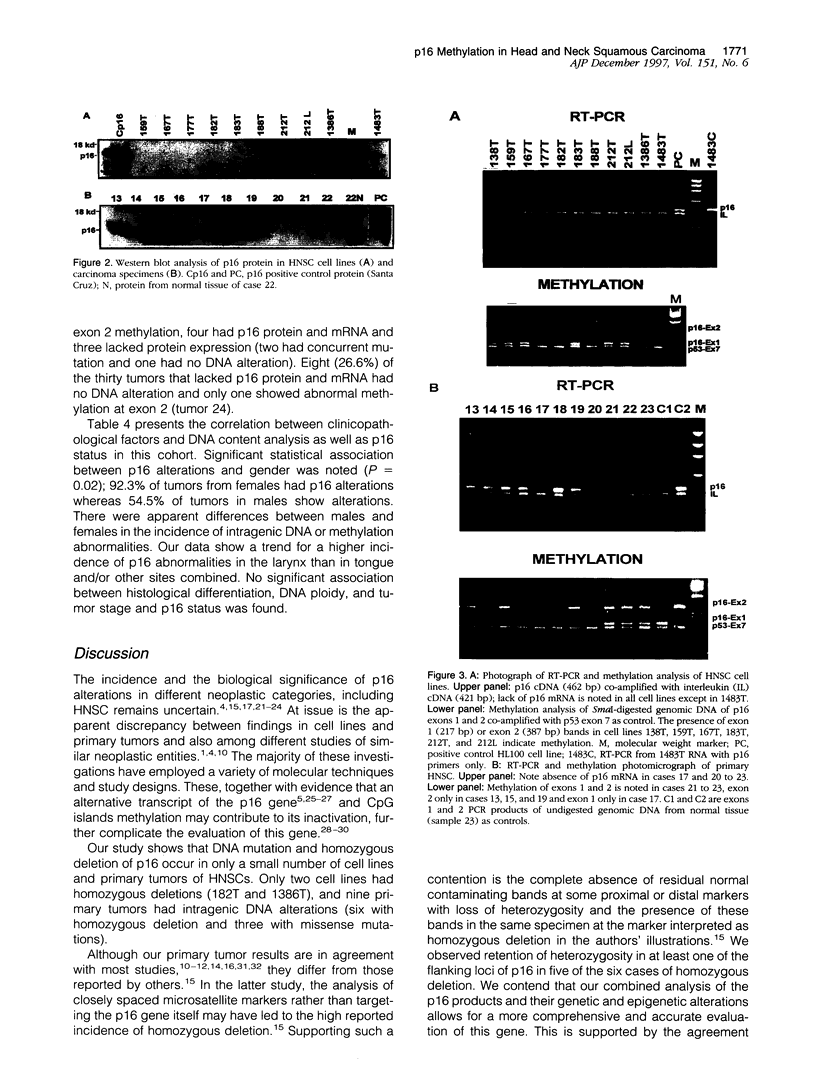
Abstract
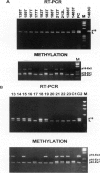
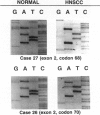
We studied 11 head and neck squamous carcinoma (HNSC) cell lines and 46 primary tumors for p16 gene status by protein, mRNA, and DNA genetic/epigenetic analyses to determine the incidence, the mechanism(s), and the potential biological significance of its inactivation. Of the 11 cell lines, only 1 showed intact p16 and 10 lacked its protein and mRNA; DNA analysis of these 10 cell lines showed 2 homozygous deletions, 6 methylations at exon 1 and 2, and 2 with no detectable abnormalities. In primary tumors, 16 (34.7%) of the 46 showed detectable p16 protein and mRNA; of these, 12 had no DNA abnormalities and 4 had only exon 2 methylation. Loss of p16 expression was found in three tumors with concurrent mutation at exon 2 and methylation at exon 2 (two) and both 1 and 2 (one). Of the 30 tumors that lacked p16 protein, 27 also lacked mRNA, 1 had detectable p16 mRNA, and 2 failed RT-PCR amplification. Twenty-two of the thirty tumors showed DNA alterations and eight manifested no abnormalities; DNA alterations comprised 6 homozygous deletions, 2 concurrent mutations and methylation of exon 2, and 13 with methylation at exon 1 and exons 1 and 2 (12 with methylation only and 1 with mutation) at exon 1. Except for patients' gender (P = 0.02), no significant correlation between p16 and clinicopathological factors was observed. We conclude that in HNSC 1) intragenic p16 alterations are infrequent events, 2) methylation of exon 1 constitutes a common mechanism in silencing the p16 gene, 3) p16 inactivation may play an important role in the early development and progression of HNSC, and 4) no association between p16 alterations and conventional clinicopathological factors was noted in this cohort.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. The essentials of DNA methylation. Cell. 1992 Jul 10;70(1):5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- Brenner A. J., Paladugu A., Wang H., Olopade O. I., Dreyling M. H., Aldaz C. M. Preferential loss of expression of p16(INK4a) rather than p19(ARF) in breast cancer. Clin Cancer Res. 1996 Dec;2(12):1993–1998. [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Cairns P., Polascik T. J., Eby Y., Tokino K., Califano J., Merlo A., Mao L., Herath J., Jenkins R., Westra W. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995 Oct;11(2):210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Foulkes W. D., Beynon G., Davis M., Englefield P. LOH and mutation analysis of CDKN2 in primary human ovarian cancers. Int J Cancer. 1995 Oct 9;63(2):222–225. doi: 10.1002/ijc.2910630213. [DOI] [PubMed] [Google Scholar]
- Enders G. H., Koh J., Missero C., Rustgi A. K., Harlow E. p16 inhibition of transformed and primary squamous epithelial cells. Oncogene. 1996 Mar 21;12(6):1239–1245. [PubMed] [Google Scholar]
- Flores J. F., Walker G. J., Glendening J. M., Haluska F. G., Castresana J. S., Rubio M. P., Pastorfide G. C., Boyer L. A., Kao W. H., Bulyk M. L. Loss of the p16INK4a and p15INK4b genes, as well as neighboring 9p21 markers, in sporadic melanoma. Cancer Res. 1996 Nov 1;56(21):5023–5032. [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Bender C. M., Yang A. S., Nguyen T., Beart R. W., Van Tornout J. M., Jones P. A. Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995 Oct 15;55(20):4531–4535. [PubMed] [Google Scholar]
- Heinzel P. A., Balaram P., Bernard H. U. Mutations and polymorphisms in the p53, p21 and p16 genes in oral carcinomas of Indian betel quid chewers. Int J Cancer. 1996 Nov 15;68(4):420–423. doi: 10.1002/(SICI)1097-0215(19961115)68:4<420::AID-IJC3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Herman J. G., Merlo A., Mao L., Lapidus R. G., Issa J. P., Davidson N. E., Sidransky D., Baylin S. B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995 Oct 15;55(20):4525–4530. [PubMed] [Google Scholar]
- Igaki H., Sasaki H., Tachimori Y., Kato H., Watanabe H., Kimura T., Harada Y., Sugimura T., Terada M. Mutation frequency of the p16/CDKN2 gene in primary cancers in the upper digestive tract. Cancer Res. 1995 Aug 1;55(15):3421–3423. [PubMed] [Google Scholar]
- Jones P. A. DNA methylation errors and cancer. Cancer Res. 1996 Jun 1;56(11):2463–2467. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Komiya A., Suzuki H., Aida S., Yatani R., Shimazaki J. Mutational analysis of CDKN2 (CDK4I/MTS1) gene in tissues and cell lines of human prostate cancer. Jpn J Cancer Res. 1995 Jul;86(7):622–625. doi: 10.1111/j.1349-7006.1995.tb02443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. J. p16INK4a: a gene with a dual capacity to encode unrelated proteins that inhibit cell cycle progression. Oncogene. 1996 May 16;12(10):2041–2044. [PubMed] [Google Scholar]
- Li Y. J., Hoang-Xuan K., Delattre J. Y., Poisson M., Thomas G., Hamelin R. Frequent loss of heterozygosity on chromosome 9, and low incidence of mutations of cyclin-dependent kinase inhibitors p15 (MTS2) and p16 (MTS1) genes in gliomas. Oncogene. 1995 Aug 3;11(3):597–600. [PubMed] [Google Scholar]
- Liggett W. H., Jr, Sewell D. A., Rocco J., Ahrendt S. A., Koch W., Sidransky D. p16 and p16 beta are potent growth suppressors of head and neck squamous carcinoma cells in vitro. Cancer Res. 1996 Sep 15;56(18):4119–4123. [PubMed] [Google Scholar]
- Lo K. W., Cheung S. T., Leung S. F., van Hasselt A., Tsang Y. S., Mak K. F., Chung Y. F., Woo J. K., Lee J. C., Huang D. P. Hypermethylation of the p16 gene in nasopharyngeal carcinoma. Cancer Res. 1996 Jun 15;56(12):2721–2725. [PubMed] [Google Scholar]
- Lo K. W., Huang D. P., Lau K. M. p16 gene alterations in nasopharyngeal carcinoma. Cancer Res. 1995 May 15;55(10):2039–2043. [PubMed] [Google Scholar]
- Mao L., Merlo A., Bedi G., Shapiro G. I., Edwards C. D., Rollins B. J., Sidransky D. A novel p16INK4A transcript. Cancer Res. 1995 Jul 15;55(14):2995–2997. [PubMed] [Google Scholar]
- Matsuda H., Konishi N., Hiasa Y., Hayashi I., Tsuzuki T., Tao M., Kitahori Y., Yoshioka N., Kirita T., Sugimura M. Alterations of p16/CDKN2, p53 and ras genes in oral squamous cell carcinomas and premalignant lesions. J Oral Pathol Med. 1996 May;25(5):232–238. doi: 10.1111/j.1600-0714.1996.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Nagatake M., Osada H., Kondo M., Uchida K., Nishio M., Shimokata K., Takahashi T., Takahashi T. Aberrant hypermethylation at the bcl-2 locus at 18q21 in human lung cancers. Cancer Res. 1996 Apr 15;56(8):1886–1891. [PubMed] [Google Scholar]
- Nakagawa K., Conrad N. K., Williams J. P., Johnson B. E., Kelley M. J. Mechanism of inactivation of CDKN2 and MTS2 in non-small cell lung cancer and association with advanced stage. Oncogene. 1995 Nov 2;11(9):1843–1851. [PubMed] [Google Scholar]
- Ohnishi H., Kawamura M., Ida K., Sheng X. M., Hanada R., Nobori T., Yamamori S., Hayashi Y. Homozygous deletions of p16/MTS1 gene are frequent but mutations are infrequent in childhood T-cell acute lymphoblastic leukemia. Blood. 1995 Aug 15;86(4):1269–1275. [PubMed] [Google Scholar]
- Otterson G. A., Khleif S. N., Chen W., Coxon A. B., Kaye F. J. CDKN2 gene silencing in lung cancer by DNA hypermethylation and kinetics of p16INK4 protein induction by 5-aza 2'deoxycytidine. Oncogene. 1995 Sep 21;11(6):1211–1216. [PubMed] [Google Scholar]
- Reed A. L., Califano J., Cairns P., Westra W. H., Jones R. M., Koch W., Ahrendt S., Eby Y., Sewell D., Nawroz H. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996 Aug 15;56(16):3630–3633. [PubMed] [Google Scholar]
- Reed J. A., Loganzo F., Jr, Shea C. R., Walker G. J., Flores J. F., Glendening J. M., Bogdany J. K., Shiel M. J., Haluska F. G., Fountain J. W. Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res. 1995 Jul 1;55(13):2713–2718. [PubMed] [Google Scholar]
- Serrano A., García A., Abril E., Garrido F., Ruiz-Cabello F. Methylated CpG points identified within MAGE-1 promoter are involved in gene repression. Int J Cancer. 1996 Nov 15;68(4):464–470. doi: 10.1002/(SICI)1097-0215(19961115)68:4<464::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Serrano M., Gómez-Lahoz E., DePinho R. A., Beach D., Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995 Jan 13;267(5195):249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Shapiro G. I., Park J. E., Edwards C. D., Mao L., Merlo A., Sidransky D., Ewen M. E., Rollins B. J. Multiple mechanisms of p16INK4A inactivation in non-small cell lung cancer cell lines. Cancer Res. 1995 Dec 15;55(24):6200–6209. [PubMed] [Google Scholar]
- Shimizu T., Sekiya T. Loss of heterozygosity at 9p21 loci and mutations of the MTS1 and MTS2 genes in human lung cancers. Int J Cancer. 1995 Nov 27;63(5):616–620. doi: 10.1002/ijc.2910630503. [DOI] [PubMed] [Google Scholar]
- Southgate J., Proffitt J., Roberts P., Smith B., Selby P. Loss of cyclin-dependent kinase inhibitor genes and chromosome 9 karyotypic abnormalities in human bladder cancer cell lines. Br J Cancer. 1995 Nov;72(5):1214–1218. doi: 10.1038/bjc.1995.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S., Jiang P., Dayananth P., Tavtigian S. V., Katcher H., Parry D., Peters G., Kamb A. Complex structure and regulation of the P16 (MTS1) locus. Cancer Res. 1995 Jul 15;55(14):2988–2994. [PubMed] [Google Scholar]
- Sun Y., Hildesheim A., Lanier A. E., Cao Y., Yao K. T., Raab-Traub N., Yang C. S. No point mutation but decreased expression of the p16/MTS1 tumor suppressor gene in nasopharyngeal carcinomas. Oncogene. 1995 Feb 16;10(4):785–788. [PubMed] [Google Scholar]
- Tamimi Y., Bringuier P. P., Smit F., van Bokhoven A., Abbas A., Debruyne F. M., Schalken J. A. Homozygous deletions of p16(INK4) occur frequently in bilharziasis-associated bladder cancer. Int J Cancer. 1996 Oct 9;68(2):183–187. doi: 10.1002/(SICI)1097-0215(19961009)68:2<183::AID-IJC7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Thoraval D., Asakawa J., Wimmer K., Kuick R., Lamb B., Richardson B., Ambros P., Glover T., Hanash S. Demethylation of repetitive DNA sequences in neuroblastoma. Genes Chromosomes Cancer. 1996 Dec;17(4):234–244. doi: 10.1002/(SICI)1098-2264(199612)17:4<234::AID-GCC5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Tsunashima K., Endo Y., Asakura H., Kanda H., Nomura K., Kitagawa T., Kominami R. A novel clonality assay for the mouse: application to hepatocellular carcinomas induced with diethylnitrosamine. Mol Carcinog. 1996 Jan;15(1):33–37. doi: 10.1002/(SICI)1098-2744(199601)15:1<33::AID-MC5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Yang R., Gombart A. F., Serrano M., Koeffler H. P. Mutational effects on the p16INK4a tumor suppressor protein. Cancer Res. 1995 Jun 15;55(12):2503–2506. [PubMed] [Google Scholar]
- Yeudall W. A., Crawford R. Y., Ensley J. F., Robbins K. C. MTS1/CDK4I is altered in cell lines derived from primary and metastatic oral squamous cell carcinoma. Carcinogenesis. 1994 Dec;15(12):2683–2686. doi: 10.1093/carcin/15.12.2683. [DOI] [PubMed] [Google Scholar]
- Zhang S. Y., Klein-Szanto A. J., Sauter E. R., Shafarenko M., Mitsunaga S., Nobori T., Carson D. A., Ridge J. A., Goodrow T. L. Higher frequency of alterations in the p16/CDKN2 gene in squamous cell carcinoma cell lines than in primary tumors of the head and neck. Cancer Res. 1994 Oct 1;54(19):5050–5053. [PubMed] [Google Scholar]