Abstract
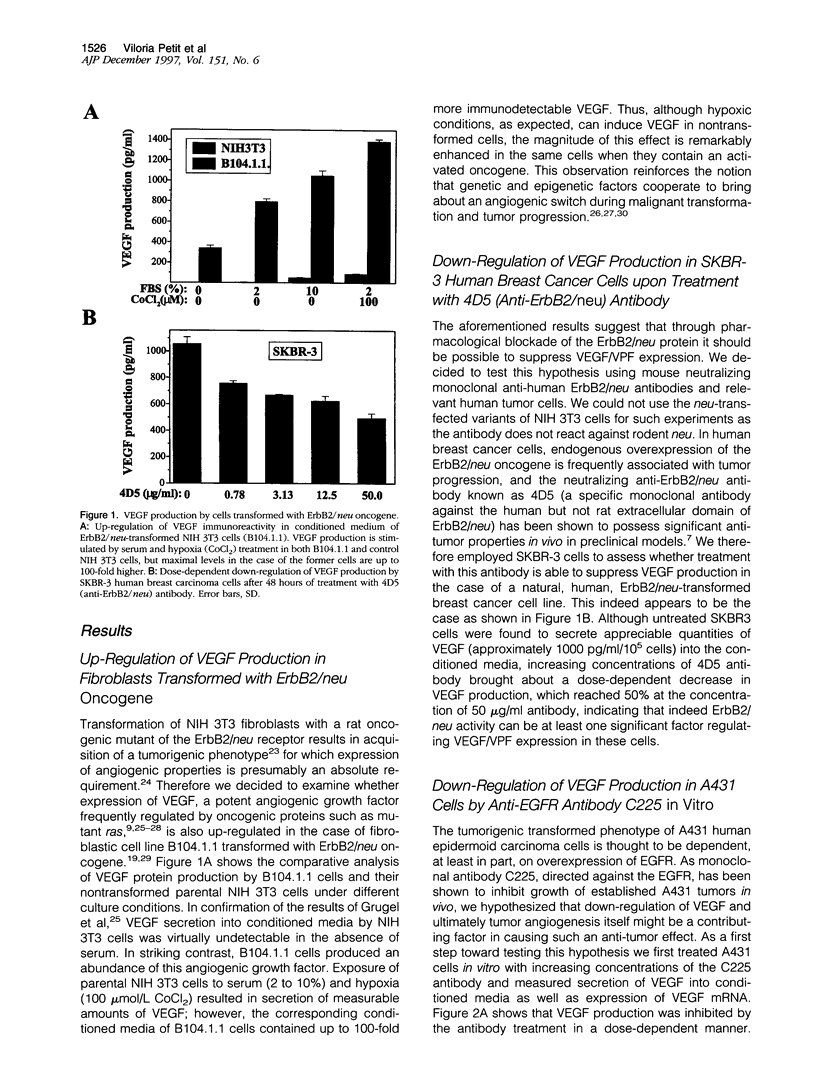
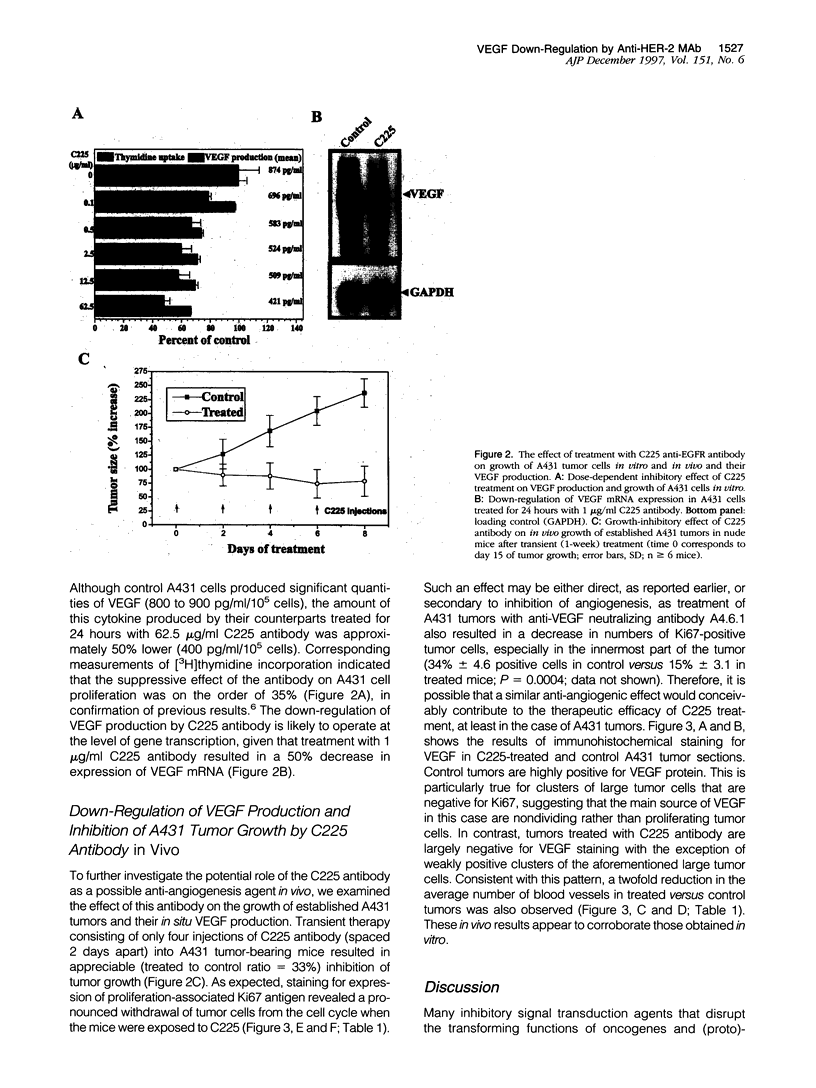
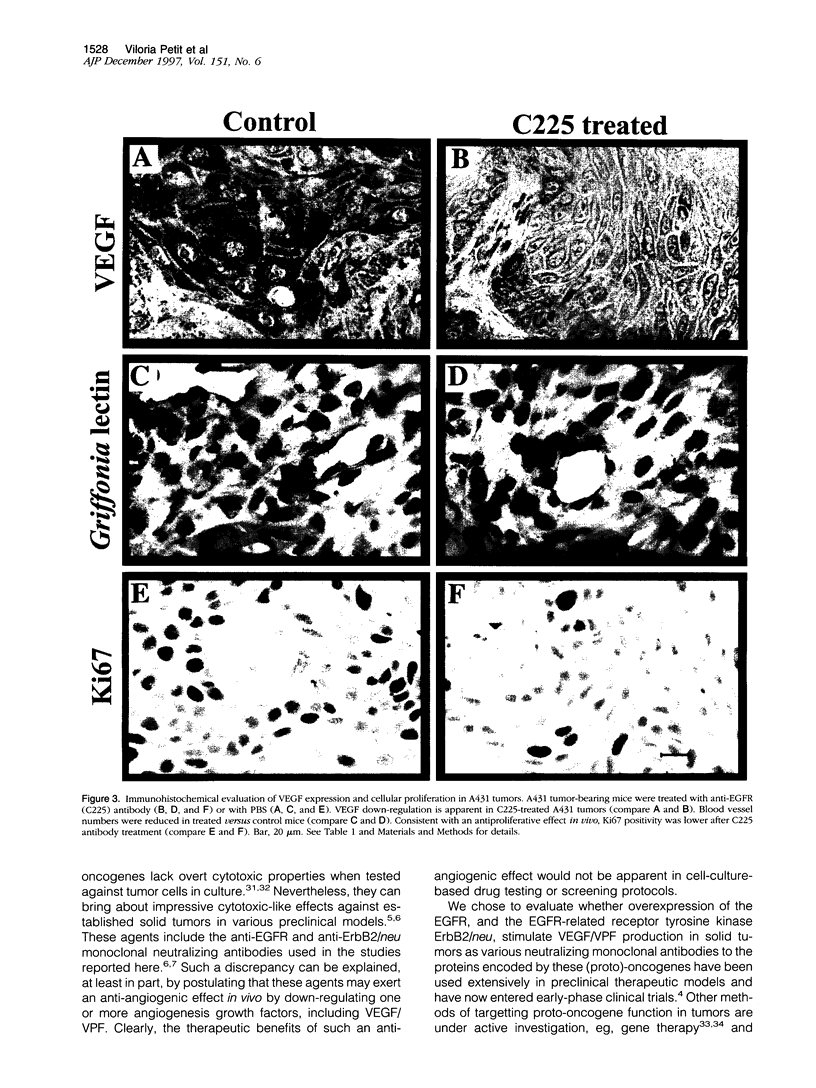
The overexpression in tumor cells of (proto)-oncogenic receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) or ErbB2/neu (also known as HER-2) is generally thought to contribute to the development of solid tumors primarily through their effects on promoting uncontrolled cell proliferation. However, agents that antagonize the function of the protein products encoded by these (proto)-oncogenes are known to behave in vivo in a cytotoxic-like manner. This implies that such oncogenes may regulate critical cell survival functions, including angiogenesis. The latter could occur as a consequence of regulation of relevant growth factors by such oncogenes. We therefore sought to determine whether EGFR or ErbB2/neu may contribute to tumor angiogenesis by examining their effects on the expression of vascular endothelial cell growth factor (VEGF)/vascular permeability factor (VPF), one of the most important of all known inducers of tumor angiogenesis. We found that in vitro treatment of EGFR-positive A431 human epidermoid carcinoma cells, which are known to be heavily dependent on VEGF/VPF in vivo as an angiogenesis growth factor, with the C225 anti-EGFR neutralizing antibody caused a dose-dependent inhibition of VEGF protein expression. Prominent suppression of VEGF/VPF expression in vivo, as well as a significant reduction in tumor blood vessel counts, were also observed in established A431 tumors shortly after injection of the antibody as few as four times into nude mice. Transformation of NIH 3T3 fibroblasts with mutant ErbB2/neu, another EGFR-like oncogenic tyrosine kinase, resulted in a significant induction of VEGF/VPF, and the magnitude of this effect was further elevated by hypoxia. Moreover, treatment of ErbB2/neu-positive SKBR-3 human breast cancer cells in vitro with a specific neutralizing anti-ErbB2/neu monoclonal antibody (4D5) resulted in a dose-dependent reduction of VEGF/VPF protein expression. Taken together, the results suggest that oncogenic properties of EGFR and ErbB2/neu may, at least in part, be mediated by stimulation of tumor angiogenesis by up-regulating potent angiogenesis growth factors such as VEGF/VPF. These genetic changes may cooperate with epigenetic/environmental effects such as hypoxia to maximally stimulate VEGF/VPF expression. Therapeutic disruption of EGFR or ErbB2/neu protein function in vivo may therefore result in partial suppression of angiogenesis, a feature that could enhance the therapeutic index of such agents in vivo and endow them with anti-tumor effects, the magnitude of which may be out of proportion with their observed cytostatic effects in monolayer tissue culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon T., Hemo I., Itin A., Pe'er J., Stone J., Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995 Oct;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Arbiser J. L., Moses M. A., Fernandez C. A., Ghiso N., Cao Y., Klauber N., Frank D., Brownlee M., Flynn E., Parangi S. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Benjamin L. E., Keshet E. Conditional switching of vascular endothelial growth factor (VEGF) expression in tumors: induction of endothelial cell shedding and regression of hemangioblastoma-like vessels by VEGF withdrawal. Proc Natl Acad Sci U S A. 1997 Aug 5;94(16):8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Stellmach V., Hsu S. C. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- Buchdunger E., Mett H., Trinks U., Regenass U., Müller M., Meyer T., Beilstein P., Wirz B., Schneider P., Traxler P. 4,5-bis(4-fluoroanilino)phthalimide: A selective inhibitor of the epidermal growth factor receptor signal transduction pathway with potent in vivo antitumor activity. Clin Cancer Res. 1995 Aug;1(8):813–821. [PubMed] [Google Scholar]
- Cheng S. Y., Huang H. J., Nagane M., Ji X. D., Wang D., Shih C. C., Arap W., Huang C. M., Cavenee W. K. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello F., Damiano V., Bianco R., Bianco C., Fontanini G., De Laurentiis M., De Placido S., Mendelsohn J., Bianco A. R., Tortora G. Antitumor activity of combined blockade of epidermal growth factor receptor and protein kinase A. J Natl Cancer Inst. 1996 Dec 4;88(23):1770–1776. doi: 10.1093/jnci/88.23.1770. [DOI] [PubMed] [Google Scholar]
- Drebin J. A., Link V. C., Stern D. F., Weinberg R. A., Greene M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985 Jul;41(3):697–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- Fendly B. M., Winget M., Hudziak R. M., Lipari M. T., Napier M. A., Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990 Mar 1;50(5):1550–1558. [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore K., Chen H., Dowd M., Lu L., O'Shea K. S., Powell-Braxton L., Hillan K. J., Moore M. W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996 Apr 4;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Gille J., Swerlick R. A., Caughman S. W. Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. 1997 Feb 17;16(4):750–759. doi: 10.1093/emboj/16.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. A., Schneider T. J. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994 Feb 11;269(6):4355–4359. [PubMed] [Google Scholar]
- Goldman C. K., Kim J., Wong W. L., King V., Brock T., Gillespie G. Y. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993 Jan;4(1):121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein N. I., Prewett M., Zuklys K., Rockwell P., Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995 Nov;1(11):1311–1318. [PubMed] [Google Scholar]
- Grugel S., Finkenzeller G., Weindel K., Barleon B., Marmé D. Both v-Ha-Ras and v-Raf stimulate expression of the vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995 Oct 27;270(43):25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989 Apr 19;81(8):570–576. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- Kawamoto T., Sato J. D., Le A., Polikoff J., Sato G. H., Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1337–1341. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Mosser S. D., deSolms S. J., Giuliani E. A., Pompliano D. L., Graham S. L., Smith R. L., Scolnick E. M., Oliff A., Gibbs J. B. Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science. 1993 Jun 25;260(5116):1934–1937. doi: 10.1126/science.8316833. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Omer C. A., Conner M. W., Anthony N. J., Davide J. P., deSolms S. J., Giuliani E. A., Gomez R. P., Graham S. L., Hamilton K. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med. 1995 Aug;1(8):792–797. doi: 10.1038/nm0895-792. [DOI] [PubMed] [Google Scholar]
- Kokai Y., Myers J. N., Wada T., Brown V. I., LeVea C. M., Davis J. G., Dobashi K., Greene M. I. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989 Jul 28;58(2):287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- Lebowitz P. F., Sakamuro D., Prendergast G. C. Farnesyl transferase inhibitors induce apoptosis of Ras-transformed cells denied substratum attachment. Cancer Res. 1997 Feb 15;57(4):708–713. [PubMed] [Google Scholar]
- Lu C., Sheehan C., Rak J. W., Chambers C. A., Hozumi N., Kerbel R. S. Endogenous interleukin 6 can function as an in vivo growth- stimulatory factor for advanced-stage human melanoma cells. Clin Cancer Res. 1996 Aug;2(8):1417–1425. [PubMed] [Google Scholar]
- Mazure N. M., Chen E. Y., Yeh P., Laderoute K. R., Giaccia A. J. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996 Aug 1;56(15):3436–3440. [PubMed] [Google Scholar]
- Mendelsohn J., Fan Z. Epidermal growth factor receptor family and chemosensitization. J Natl Cancer Inst. 1997 Mar 5;89(5):341–343. doi: 10.1093/jnci/89.5.341. [DOI] [PubMed] [Google Scholar]
- Millauer B., Longhi M. P., Plate K. H., Shawver L. K., Risau W., Ullrich A., Strawn L. M. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996 Apr 1;56(7):1615–1620. [PubMed] [Google Scholar]
- Monia B. P., Johnston J. F., Geiger T., Muller M., Fabbro D. Antitumor activity of a phosphorothioate antisense oligodeoxynucleotide targeted against C-raf kinase. Nat Med. 1996 Jun;2(6):668–675. doi: 10.1038/nm0696-668. [DOI] [PubMed] [Google Scholar]
- Padhy L. C., Shih C., Cowing D., Finkelstein R., Weinberg R. A. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982 Apr;28(4):865–871. doi: 10.1016/0092-8674(82)90065-4. [DOI] [PubMed] [Google Scholar]
- Rak J., Filmus J., Kerbel R. S. Reciprocal paracrine interactions between tumour cells and endothelial cells: the 'angiogenesis progression' hypothesis. Eur J Cancer. 1996 Dec;32A(14):2438–2450. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R. S. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995 Oct 15;55(20):4575–4580. [PubMed] [Google Scholar]
- Rak J., Mitsuhashi Y., Erdos V., Huang S. N., Filmus J., Kerbel R. S. Massive programmed cell death in intestinal epithelial cells induced by three-dimensional growth conditions: suppression by mutant c-H-ras oncogene expression. J Cell Biol. 1995 Dec;131(6 Pt 1):1587–1598. doi: 10.1083/jcb.131.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury D. F., Tseng A., Jr, Fu K. K., Weinburg V., Weidner N. New prognostic factors in nasopharyngeal carcinoma. Tumor angiogenesis and C-erbB2 expression. Cancer. 1996 Apr 15;77(8):1419–1426. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1419::AID-CNCR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Brandt R., Ciardiello F., Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995 Jul;19(3):183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- Tokuda Y., Ohnishi Y., Shimamura K., Iwasawa M., Yoshimura M., Ueyama Y., Tamaoki N., Tajima T., Mitomi T. In vitro and in vivo anti-tumour effects of a humanised monoclonal antibody against c-erbB-2 product. Br J Cancer. 1996 Jun;73(11):1362–1365. doi: 10.1038/bjc.1996.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert O. V., Dameron K. M., Bouck N. Sequential development of an angiogenic phenotype by human fibroblasts progressing to tumorigenicity. Oncogene. 1997 Mar 27;14(12):1495–1502. doi: 10.1038/sj.onc.1200977. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu D., Xia W., Hung M. C. HER-2/neu-targeting cancer therapy via adenovirus-mediated E1A delivery in an animal model. Oncogene. 1995 May 18;10(10):1947–1954. [PubMed] [Google Scholar]





