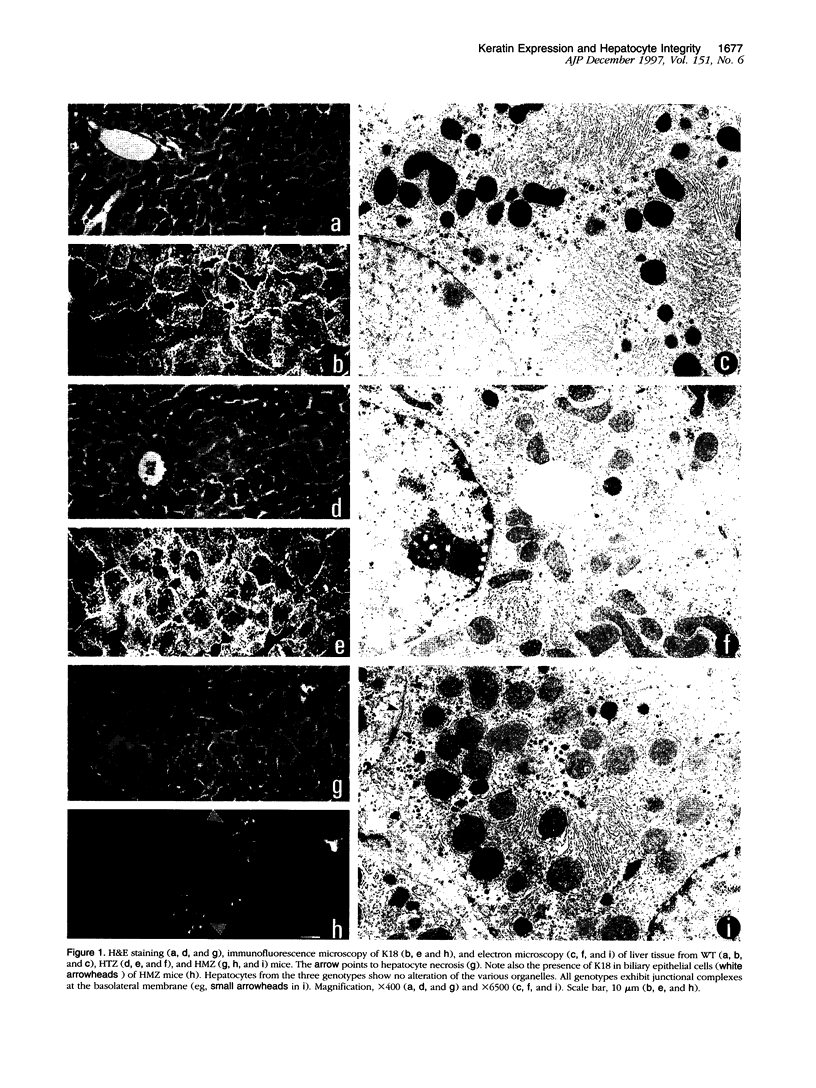
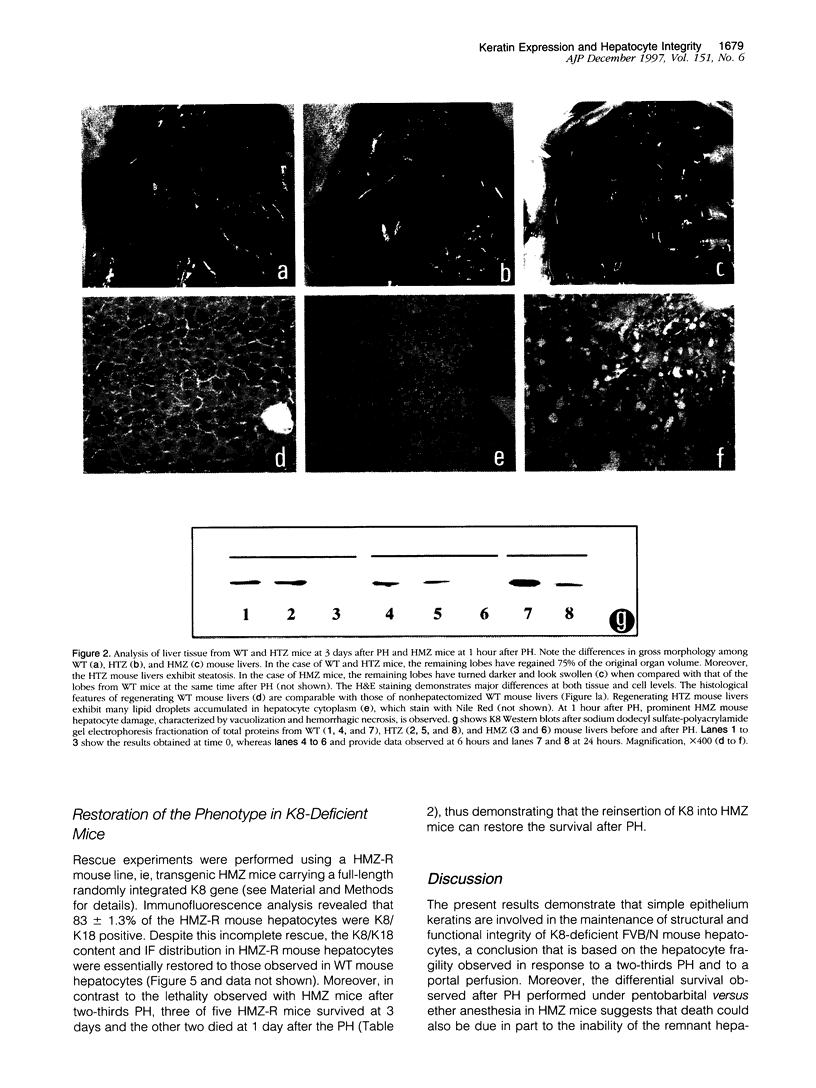
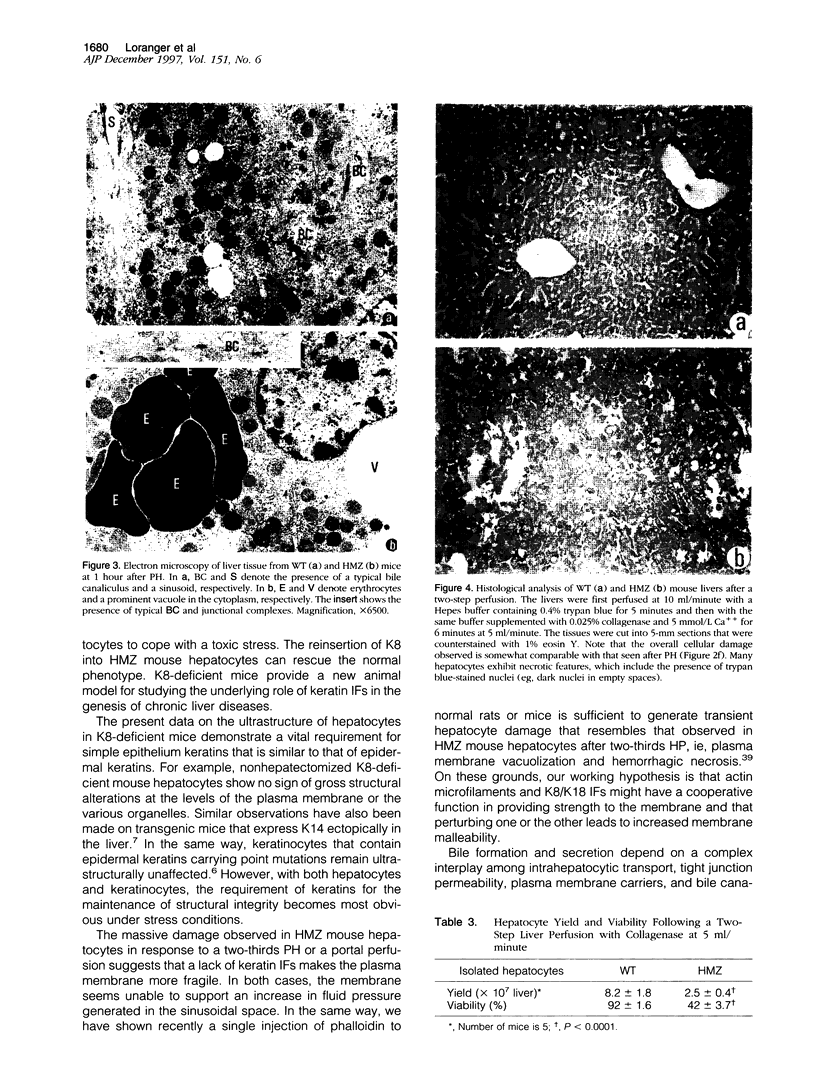
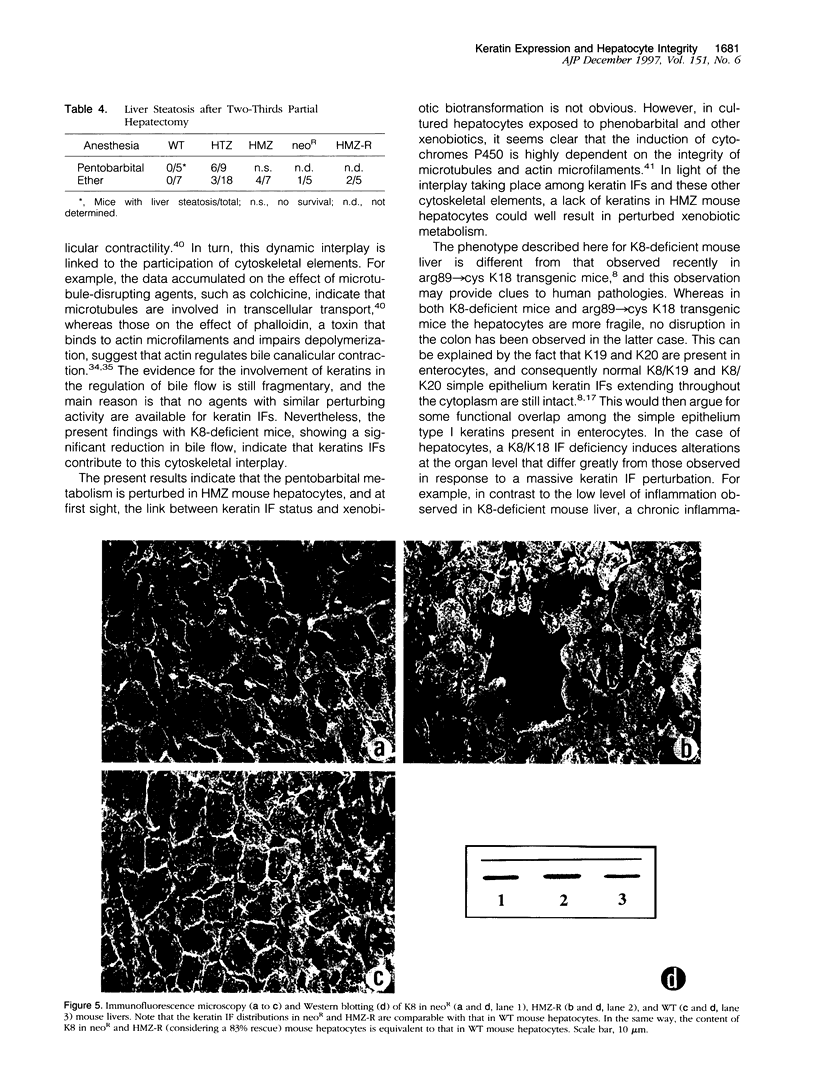
Abstract
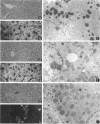
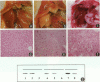
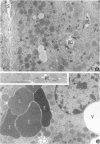
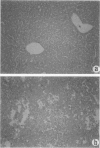
Keratin 8 (K8)-deficient adult mice develop a severe disease of the gastrointestinal tract characterized mainly by colorectal hyperplasia and inflammation. Given that hepatocytes contain K8/K18 heteropolymers only, this animal model was used to assess the contribution of these simple epithelium keratins to hepatocyte structural and functional integrity. Homozygous mutant (HMZ), heterozygous, and wild-type (WT) mice were examined for hepatocyte structural and metabolic features and their survival to partial hepatectomy. Except for the presence of few necrotic foci, no other tissular or cellular alterations were observed in nonhepatectomized HMZ mouse livers; glycogen and lipid peroxidation levels were essentially normal, but a small reduction in bile flow was observed. In response to a single pentobarbital injection, HMZ mice had longer sleeping times than heterozygous and WT mice. After a two-thirds partial hepatectomy under pentobarbital anesthesia, all HMZ mice died within a few hours, whereas those anesthetized with ether survived for 1 to 2 days. One hour after hepatectomy after pentobarbital anesthesia, many hepatocytes contained erythrocytes and large vacuoles in the cytoplasm, which suggests damage at the plasma membrane level in response to a sudden increase in portal blood flow. In line with these findings, an uptake of trypan blue by HMZ but not WT mouse hepatocytes was observed during a 10 ml/minute perfusion via the portal vein with a dye-supplemented buffer. Subsequent cellular dispersion led to viable WT mouse hepatocytes but largely nonviable HMZ mouse hepatocytes. Better viability was obtained at lower perfusion rates. Partially hepatectomized heterozygous mice developed liver steatosis, a condition that was not associated with a change in K8 content but perhaps linked to the presence of the neo gene. Transgenic HMZ mouse rescue experiments with a full-length K8 gene confirmed that the phenotypic alterations observed in partially hepatectomized HMZ mice were caused by the disruption of the K8 gene. Taken together, these findings demonstrate that simple epithelium keratins are essential for the maintenance of hepatocyte structural and functional integrity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K. M., Davis F. E., Perrone T. N., Lee E. Y., Liu Y., Vore M. Expression of an epidermal keratin protein in liver of transgenic mice causes structural and functional abnormalities. J Cell Biol. 1995 Jan;128(1-2):157–169. doi: 10.1083/jcb.128.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias I. M., Che M., Gatmaitan Z., Leveille C., Nishida T., St Pierre M. The biology of the bile canaliculus, 1993. Hepatology. 1993 Feb;17(2):318–329. [PubMed] [Google Scholar]
- Baribault H., Penner J., Iozzo R. V., Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994 Dec 15;8(24):2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- Blouin R., Blouin M. J., Royal I., Grenier A., Roop D. R., Loranger A., Marceau N. Cytokeratin 14 expression in rat liver cells in culture and localization in vivo. Differentiation. 1992 Dec;52(1):45–54. doi: 10.1111/j.1432-0436.1992.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Brixová E., Dzúriková V. Determination of glycogen, lipids and proteins in hepatic needle biopsy. Clin Chim Acta. 1972 Feb;36(2):543–548. doi: 10.1016/0009-8981(72)90032-0. [DOI] [PubMed] [Google Scholar]
- Deschenes J., Valet J. P., Marceau N. Hepatocytes from newborn and weanling rats in monolayer culture: isolation by perfusion, fibronectin-mediated adhesion, spreading, and functional activities. In Vitro. 1980 Aug;16(8):722–730. doi: 10.1007/BF02619202. [DOI] [PubMed] [Google Scholar]
- Fausto N., Webber E. M. Mechanisms of growth regulation in liver regeneration and hepatic carcinogenesis. Prog Liver Dis. 1993;11:115–137. [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Greeve M., Ferrell L., Kim M., Combs C., Roberts J., Ascher N., Wright T. L. Cirrhosis of undefined pathogenesis: absence of evidence for unknown viruses or autoimmune processes. Hepatology. 1993 Apr;17(4):593–598. doi: 10.1002/hep.1840170411. [DOI] [PubMed] [Google Scholar]
- KING H., HAWTOF D. B. Accidental intra-arterial injection of ether. JAMA. 1963 Apr 20;184:241–242. doi: 10.1001/jama.1963.73700160012021c. [DOI] [PubMed] [Google Scholar]
- Kawahara H., French S. W. Role of cytoskeleton in canalicular contraction in cultured differentiated hepatocytes. Am J Pathol. 1990 Mar;136(3):521–532. [PMC free article] [PubMed] [Google Scholar]
- Ku N. O., Michie S., Oshima R. G., Omary M. B. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J Cell Biol. 1995 Dec;131(5):1303–1314. doi: 10.1083/jcb.131.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku N. O., Wright T. L., Terrault N. A., Gish R., Omary M. B. Mutation of human keratin 18 in association with cryptogenic cirrhosis. J Clin Invest. 1997 Jan 1;99(1):19–23. doi: 10.1172/JCI119127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loranger A., Barriault C., Yousef I. M., Tuchweber B. Structural and functional alterations of hepatocytes during transient phalloidin-induced cholestasis in the rat. Toxicol Appl Pharmacol. 1996 Mar;137(1):100–111. doi: 10.1006/taap.1996.0061. [DOI] [PubMed] [Google Scholar]
- Loranger A., Tuchweber B., Gicquaud C., St-Pierre S., Côté M. G. Toxicity of peptides of Amanita virosa mushrooms in mice. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 1):1144–1152. doi: 10.1016/0272-0590(85)90151-4. [DOI] [PubMed] [Google Scholar]
- Loranger A., Tuchweber B., Youseff I., Marceau N. Biliary secretion and actin-cytokeratin filament distribution in rat hepatocytes during phalloidin-induced cholestasis. Biochem Cell Biol. 1995 Sep-Oct;73(9-10):641–649. doi: 10.1139/o95-071. [DOI] [PubMed] [Google Scholar]
- Marceau N. Epithelial cell lineages in developing, restoring, and transforming liver: evidence for the existence of a 'differentiation window'. Gut. 1994 Mar;35(3):294–296. doi: 10.1136/gut.35.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau N., Loranger A. Cytokeratin expression, fibrillar organization, and subtle function in liver cells. Biochem Cell Biol. 1995 Sep-Oct;73(9-10):619–625. doi: 10.1139/o95-068. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- McLean W. H., Lane E. B. Intermediate filaments in disease. Curr Opin Cell Biol. 1995 Feb;7(1):118–125. doi: 10.1016/0955-0674(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Michel M., Török N., Godbout M. J., Lussier M., Gaudreau P., Royal A., Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996 May;109(Pt 5):1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978 May;86(1):271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Keilin's respiratory chain concept and its chemiosmotic consequences. Science. 1979 Dec 7;206(4423):1148–1159. doi: 10.1126/science.388618. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Ku N. O. Intermediate filament proteins of the liver: emerging disease association and functions. Hepatology. 1997 May;25(5):1043–1048. doi: 10.1002/hep.510250537. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Baribault H., Caulín C. Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev. 1996 Dec;15(4):445–471. doi: 10.1007/BF00054012. [DOI] [PubMed] [Google Scholar]
- Oshima R. G. Intermediate filament molecular biology. Curr Opin Cell Biol. 1992 Feb;4(1):110–116. doi: 10.1016/0955-0674(92)90067-m. [DOI] [PubMed] [Google Scholar]
- Rossant J., Joyner A. L. Towards a molecular-genetic analysis of mammalian development. Trends Genet. 1989 Aug;5(8):277–283. doi: 10.1016/0168-9525(89)90102-9. [DOI] [PubMed] [Google Scholar]
- Rosser B. G., Gores G. J. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995 Jan;108(1):252–275. doi: 10.1016/0016-5085(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Royal I., Gourdeau H., Blouin R., Marceau N. Down-regulation of cytokeratin 14 mRNA in polyoma virus middle T-transformed rat liver epithelial cells. Cell Growth Differ. 1992 Sep;3(9):589–596. [PubMed] [Google Scholar]
- Royal I., Raptis L., Druker B. J., Marceau N. Down-regulation of cytokeratin 14 gene expression by the polyoma virus middle T antigen is dependent on c-Src association but independent of full transformation in rat liver nonparenchymal epithelial cells. Cell Growth Differ. 1996 Jun;7(6):737–743. [PubMed] [Google Scholar]
- Shiojiri N., Lemire J. M., Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991 May 15;51(10):2611–2620. [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M. Structure, function, and dynamics of keratin intermediate filaments. J Invest Dermatol. 1993 Jun;100(6):729–734. doi: 10.1111/1523-1747.ep12475665. [DOI] [PubMed] [Google Scholar]
- Tsukada N., Ackerley C. A., Phillips M. J. The structure and organization of the bile canalicular cytoskeleton with special reference to actin and actin-binding proteins. Hepatology. 1995 Apr;21(4):1106–1113. [PubMed] [Google Scholar]
- Vasseur M., Duprey P., Brûlet P., Jacob F. One gene and one pseudogene for the cytokeratin endo A. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1155–1159. doi: 10.1073/pnas.82.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]