Abstract
We have investigated the acute lung toxicity of urban particulate matter in interaction with ozone. Rats were exposed for 4 hours to clean air, ozone (0.8 ppm), the urban dust EHC-93 (5 mg/m3 or 50 mg/m3), or ozone in combination with urban dust. The animals were returned to clean air for 32 hours and then injected (intraperitoneally) with [3H]thymidine to label proliferating cells and killed after 90 minutes. The lungs were fixed by inflation, embedded in glycol methacrylate, and processed for light microscopy autoradiography. Cell labeling was low in bronchioles (0.14 +/- 0.04%) and parenchyma (0.13 +/- 0.02%) of air control animals. Inhalation of EHC-93 alone did not induce cell labeling. Ozone alone increased (P < 0.05) cell labeling (bronchioles, 0.42 +/- 0.16%; parenchyma, 0.57 +/- 0.21%), in line with an acute reparative cell proliferation. The effects of ozone were clearly potentiated by co-exposure with either the low (3.31 +/- 0.31%; 0.99 +/- 0.18%) or the high (4.45 +/- 0.51%; 1.47 +/- 0.18%) concentrations of urban dust (ozone X EHC-93, P < 0.05). Cellular changes were most notable in the epithelia of terminal bronchioles and alveolar ducts and did not distribute to the distal parenchyma. Enhanced DNA synthesis indicates that particulate matter from ambient air can exacerbate epithelial lesions in the lungs. This may extend beyond air pollutant interactions, such as to effects of inhaled particles in the lungs of compromised individuals.
Full text
PDF


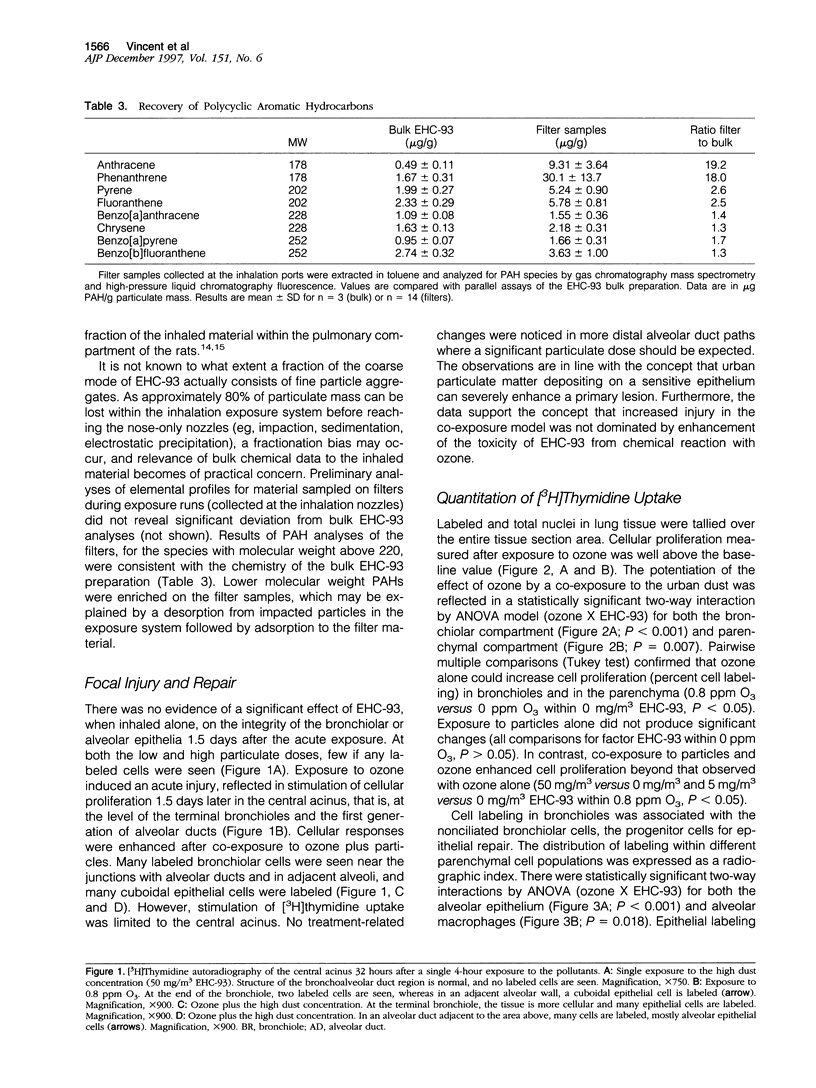
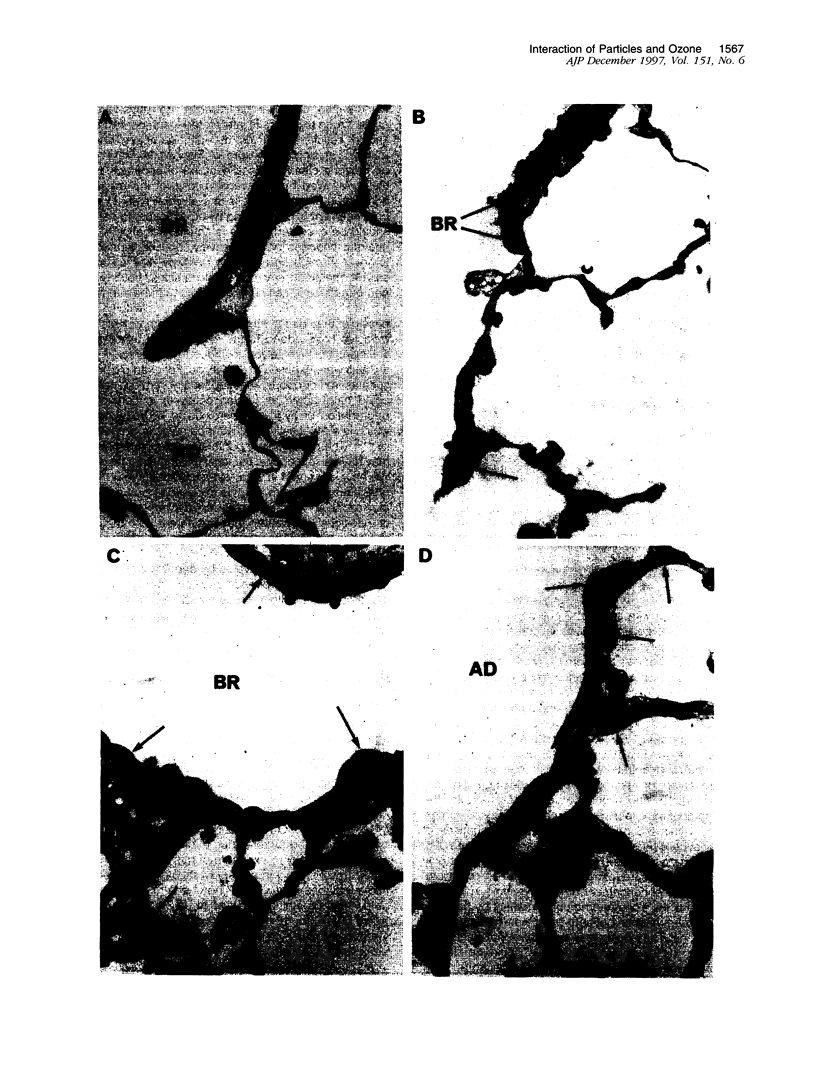



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Hedgecock C. Patterns of particle deposition and retention after instillation to mouse lung during acute injury and fibrotic repair. Exp Lung Res. 1995 Sep-Oct;21(5):695–709. doi: 10.3109/01902149509050837. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Prieditis H. L. Response of mouse lung to carbon deposition during injury and repair. Environ Health Perspect. 1995 Jan;103(1):72–76. doi: 10.1289/ehp.9510372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D. V., Sizto R. Relationship between air pollutant levels and hospital admissions in Southern Ontario. Can J Public Health. 1983 Mar-Apr;74(2):117–122. [PubMed] [Google Scholar]
- Burnett R. T., Dales R. E., Raizenne M. E., Krewski D., Summers P. W., Roberts G. R., Raad-Young M., Dann T., Brook J. Effects of low ambient levels of ozone and sulfates on the frequency of respiratory admissions to Ontario hospitals. Environ Res. 1994 May;65(2):172–194. doi: 10.1006/enrs.1994.1030. [DOI] [PubMed] [Google Scholar]
- Burnett R. T., Dales R., Krewski D., Vincent R., Dann T., Brook J. R. Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am J Epidemiol. 1995 Jul 1;142(1):15–22. doi: 10.1093/oxfordjournals.aje.a117540. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Tyler W. S., Dungworth D. L. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol. 1977 Jun;26(3):384–400. doi: 10.1016/0014-4800(77)90041-7. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Dekker N. P., Cabral-Anderson L. J., Freeman G. Quantitation of damage to the alveolar epithelium by means of type 2 cell proliferation. Am Rev Respir Dis. 1978 Oct;118(4):787–790. doi: 10.1164/arrd.1978.118.4.787. [DOI] [PubMed] [Google Scholar]
- Liu L., Kumarathasan P., Guénette J., Vincent R. Hydroxylation of salicylate in lungs of Fischer 344 rats: effects of aging and ozone exposure. Am J Physiol. 1996 Dec;271(6 Pt 1):L995–1003. doi: 10.1152/ajplung.1996.271.6.L995. [DOI] [PubMed] [Google Scholar]
- Mercer R. R., Anjilvel S., Miller F. J., Crapo J. D. Inhomogeneity of ventilatory unit volume and its effects on reactive gas uptake. J Appl Physiol (1985) 1991 May;70(5):2193–2205. doi: 10.1152/jappl.1991.70.5.2193. [DOI] [PubMed] [Google Scholar]
- Møller M., Alfheim I. Mutagenicity and PAH-analysis of airborne particulate matter. Atmos Environ. 1980;14(1):83–88. doi: 10.1016/0004-6981(80)90111-0. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and daily mortality: a review and meta analysis. Environ Res. 1994 Jan;64(1):36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- Vedal S. Ambient particles and health: lines that divide. J Air Waste Manag Assoc. 1997 May;47(5):551–581. doi: 10.1080/10473289.1997.10463922. [DOI] [PubMed] [Google Scholar]
- Vincent R., Adamson I. Y. Cellular kinetics in the lungs of aging Fischer 344 rats after acute exposure to ozone. Am J Pathol. 1995 Apr;146(4):1008–1016. [PMC free article] [PubMed] [Google Scholar]
- Vincent R., Goegan P., Johnson G., Brook J. R., Kumarathasan P., Bouthillier L., Burnett R. T. Regulation of promoter-CAT stress genes in HepG2 cells by suspensions of particles from ambient air. Fundam Appl Toxicol. 1997 Sep;39(1):18–32. doi: 10.1006/faat.1997.2336. [DOI] [PubMed] [Google Scholar]
- Vincent R., Janzen E. G., Chen G., Kumarathasan P., Haire D. L., Guénette J., Chen J. Z., Bray T. M. Spin trapping study in the lungs and liver of F344 rats after exposure to ozone. Free Radic Res. 1996 Dec;25(6):475–488. doi: 10.3109/10715769609149070. [DOI] [PubMed] [Google Scholar]