Abstract
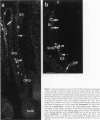
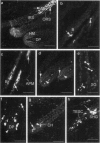
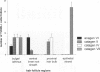
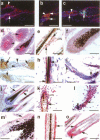
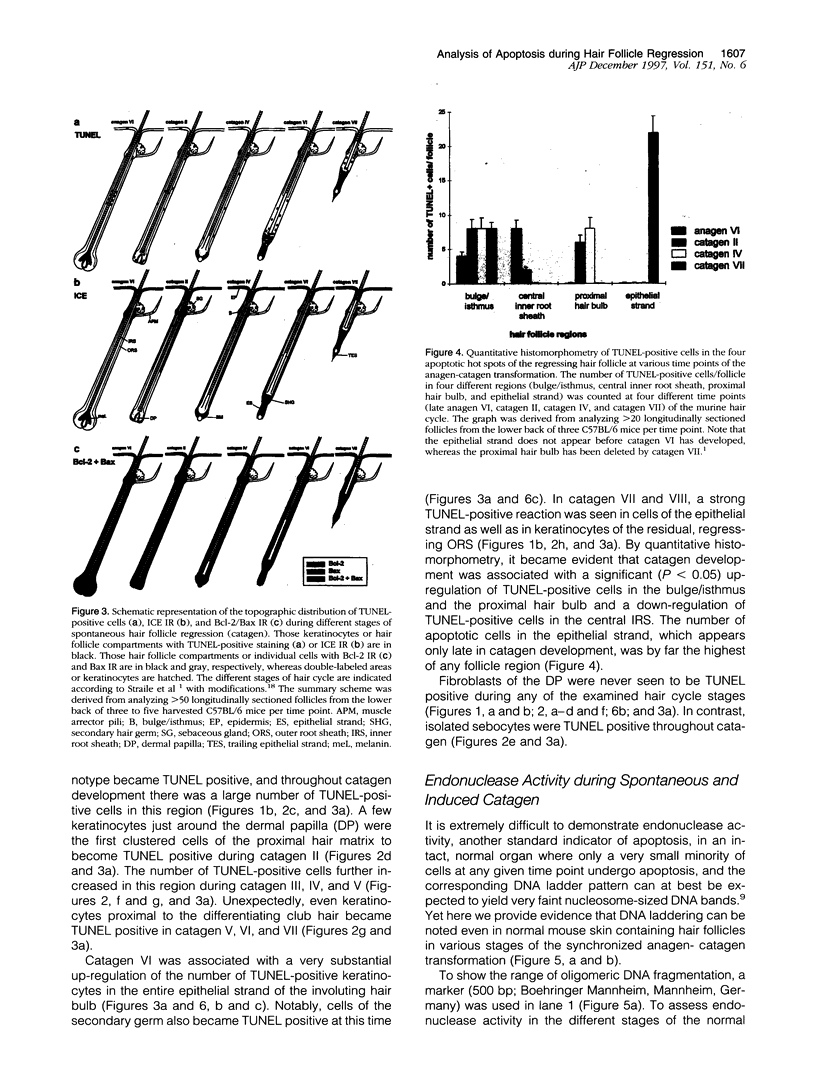
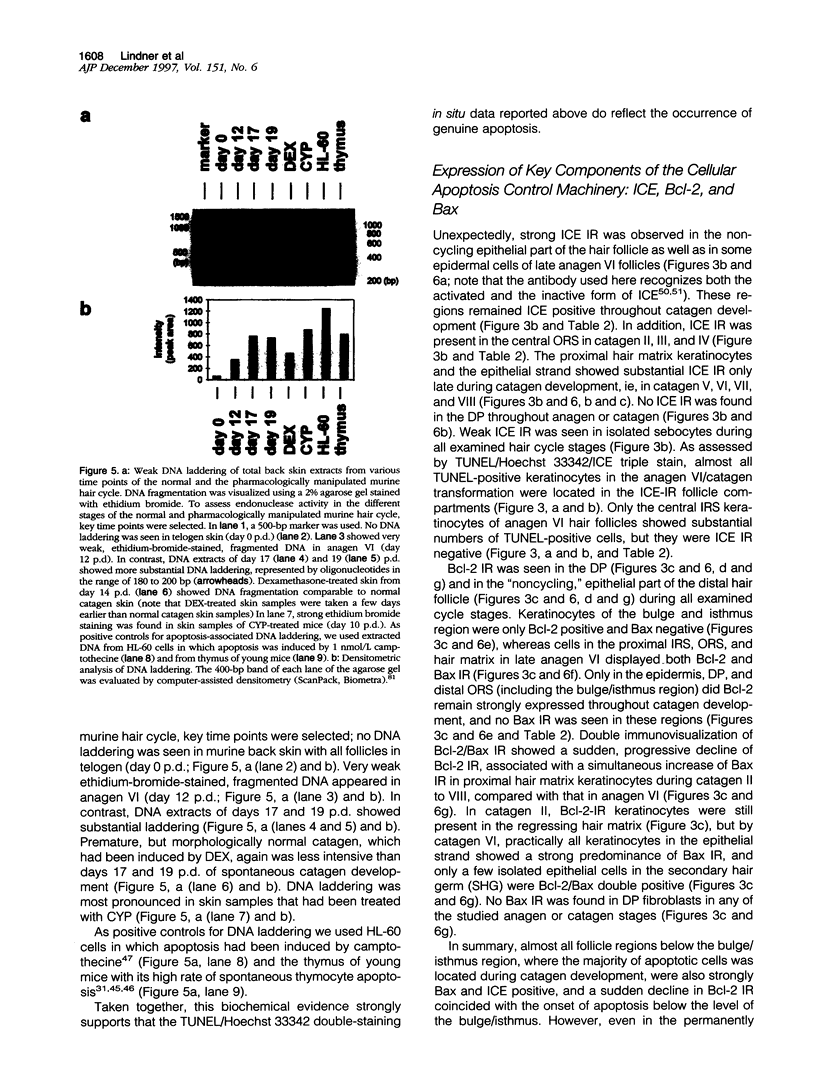
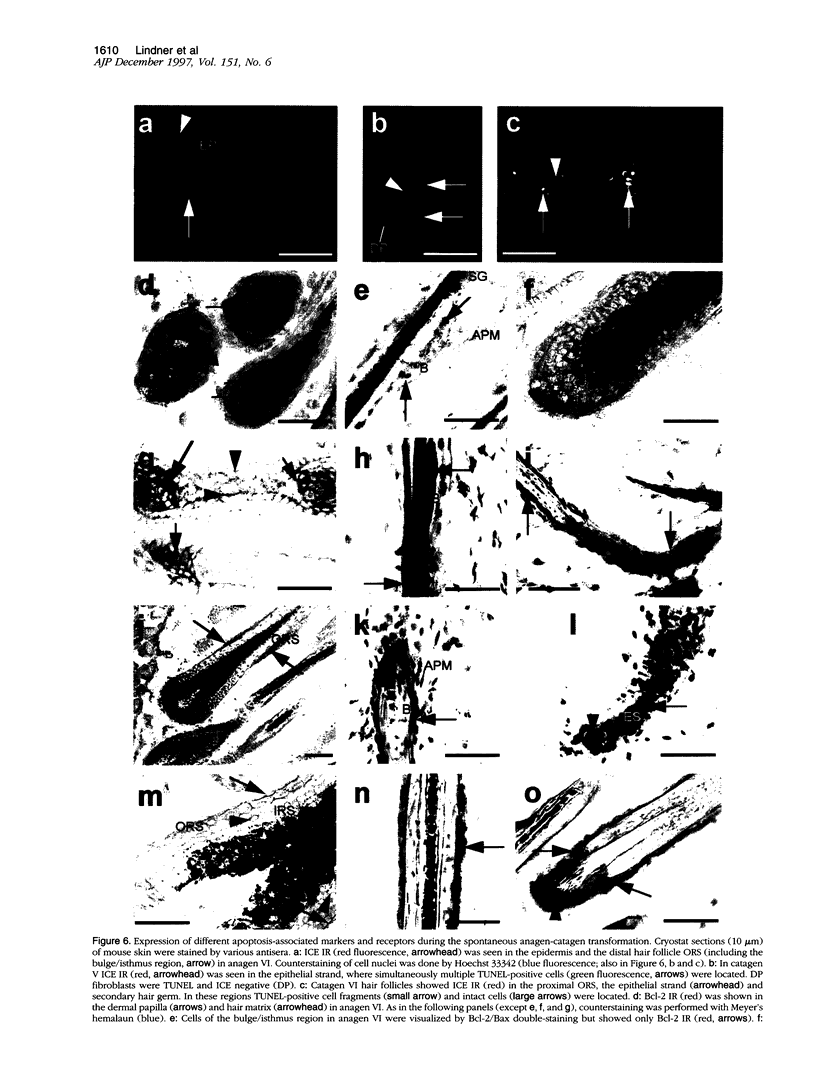
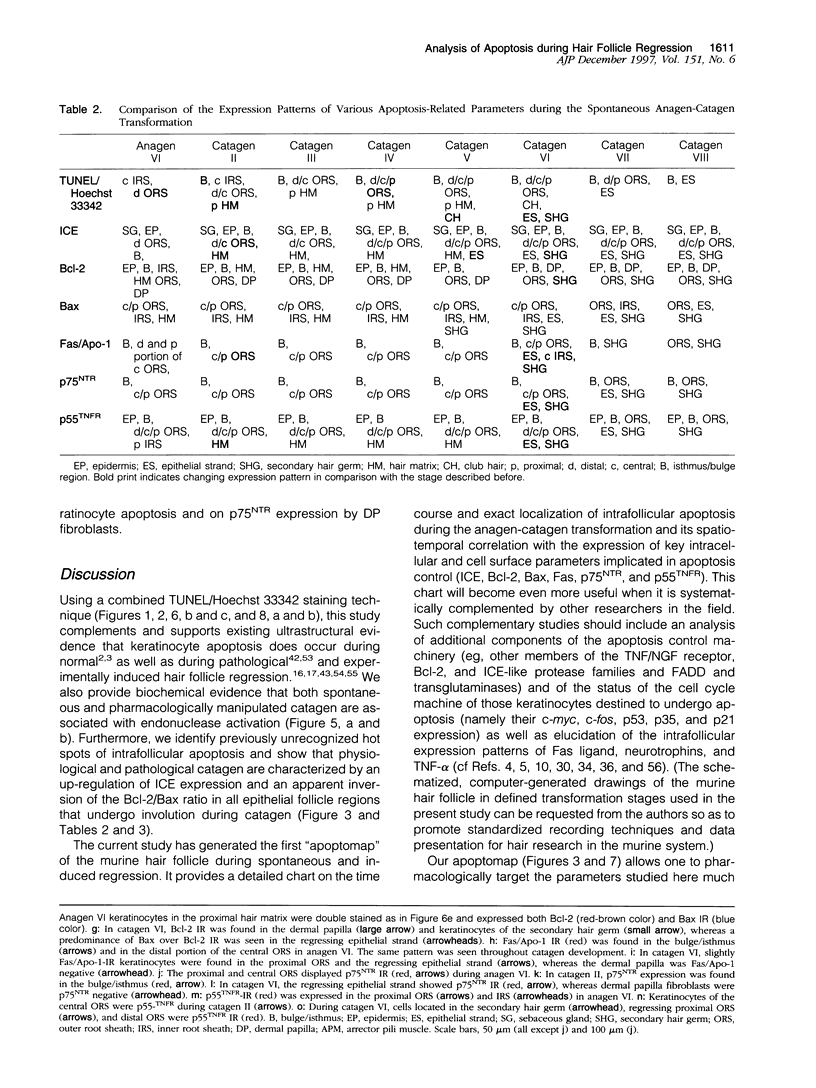
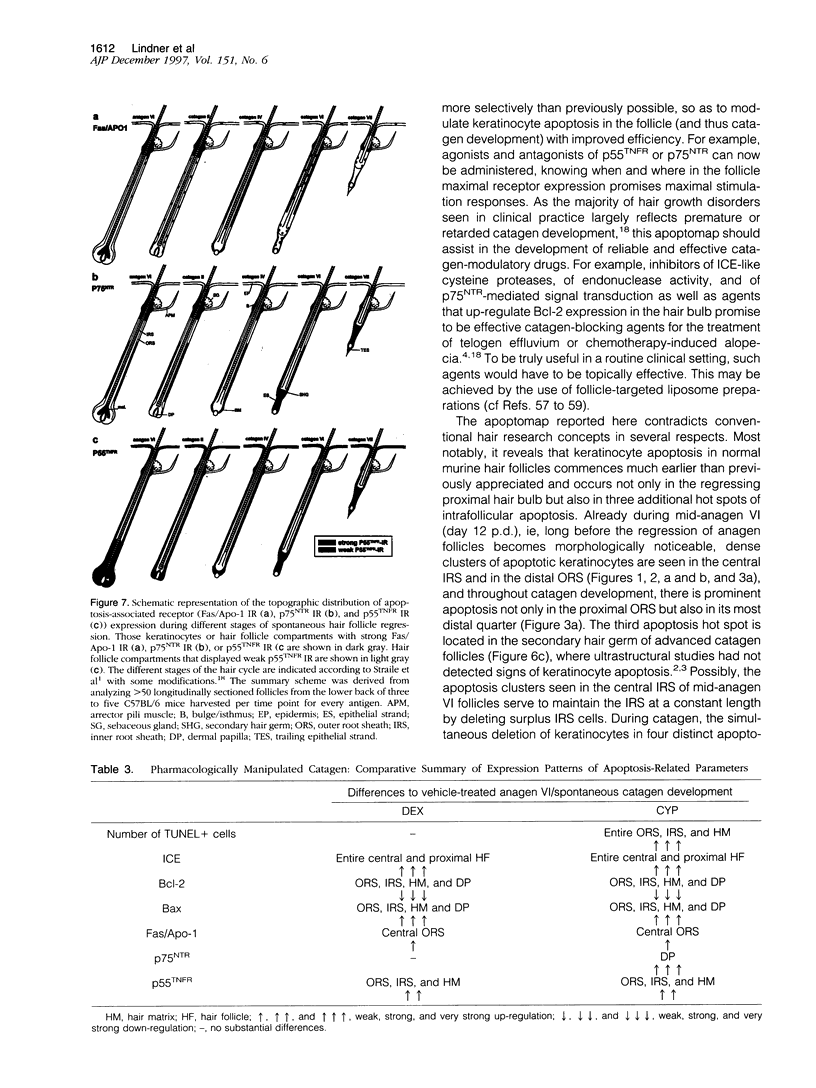
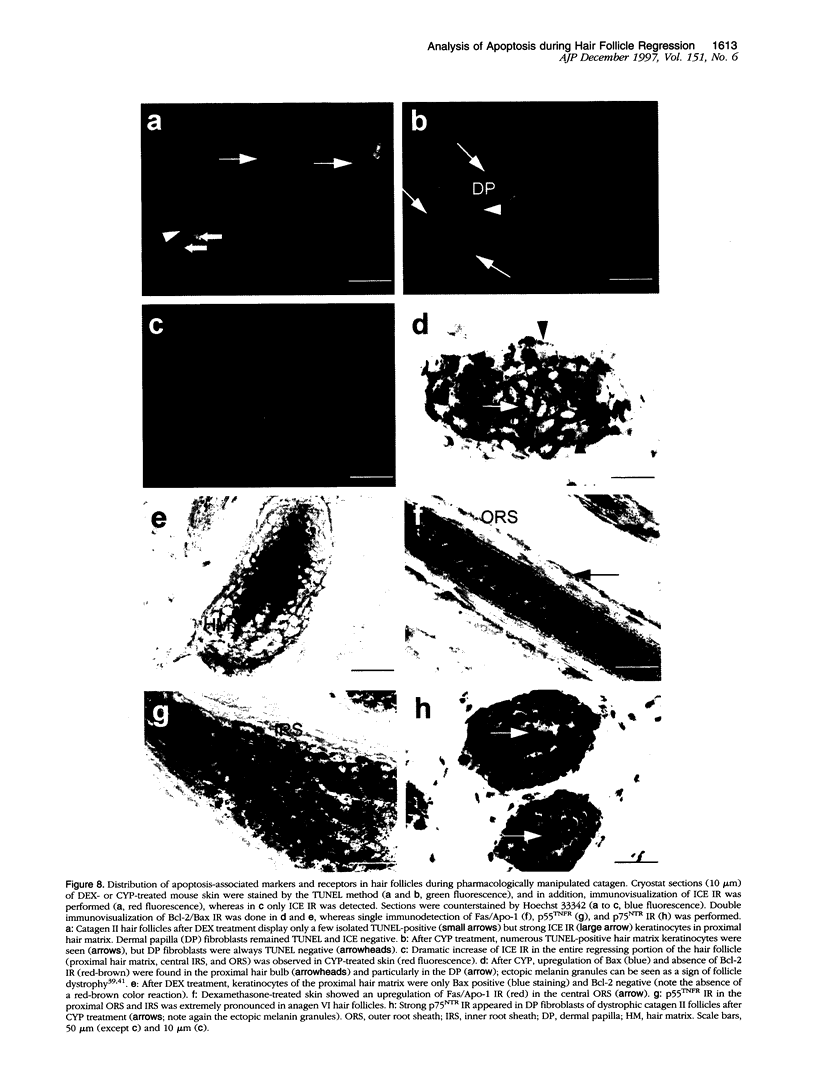
Keratinocyte apoptosis is a central element in the regulation of hair follicle regression (catagen), yet the exact location and the control of follicular keratinocyte apoptosis remain obscure. To generate an "apoptomap" of the hair follicle, we have studied selected apoptosis-associated parameters in the C57BL/6 mouse model for hair research during normal and pharmacologically manipulated, pathological catagen development. As assessed by terminal deoxynucleotide transferase dUTP fluorescein nick end-labeling (TUNEL) stain, apoptotic cells not only appeared in the regressing proximal follicle epithelium but, surprisingly, were also seen in the central inner root sheath, in the bulge/isthmus region, and in the secondary germ, but never in the dermal papilla. These apoptosis hot spots during catagen development correlated largely with a down-regulation of the Bcl-2/Bax ratio but only poorly with the expression patterns of interleukin-1beta converting enzyme, p55TNFR, and Fas/Apo-1 immunoreactivity. Instead, a higher correlation was found with p75NTR expression. During cyclophosphamide-induced follicle dystrophy and alopecia, massive keratinocyte apoptosis occurred in the entire proximal hair bulb, except in the dermal papilla, despite a strong up-regulation of Bax and p75NTR immunoreactivity. Selected receptors of the tumor necrosis factor/nerve growth factor family and members of the Bcl-2 family may also play a key role in the control of follicular keratinocyte apoptosis in situ.
Full text
PDF


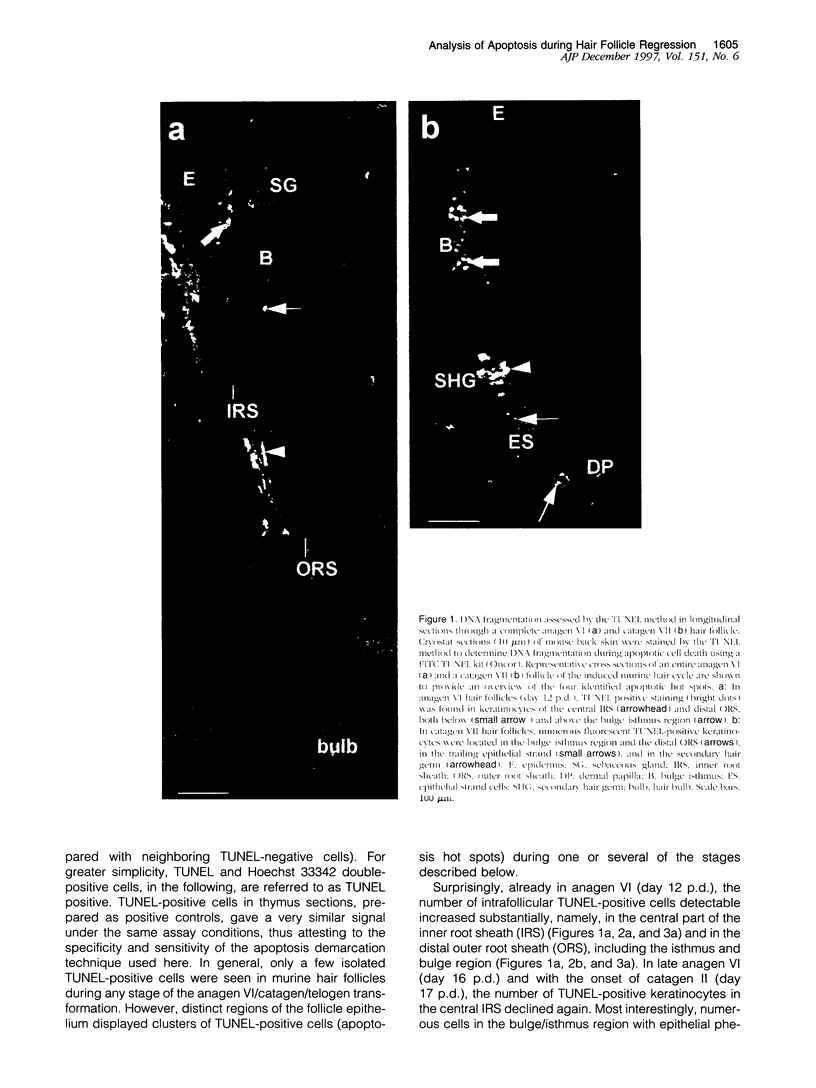
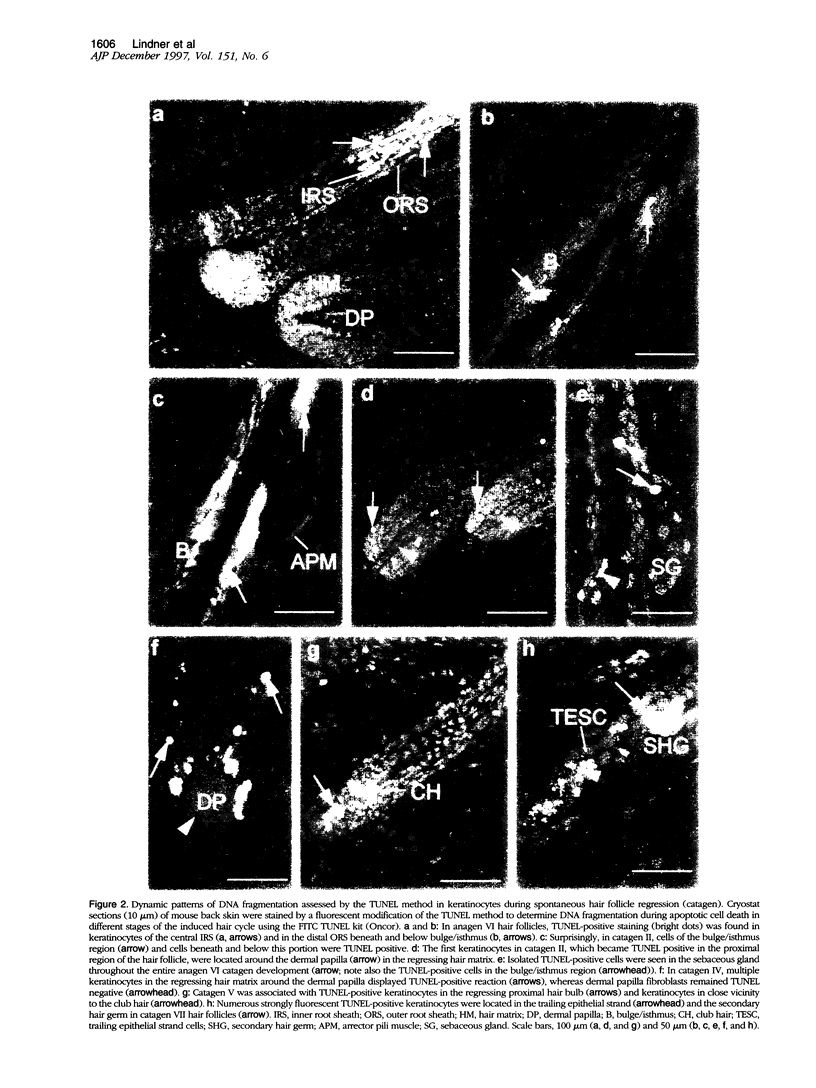





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997 Feb;18(2):72–76. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- Bhat R. V., DiRocco R., Marcy V. R., Flood D. G., Zhu Y., Dobrzanski P., Siman R., Scott R., Contreras P. C., Miller M. Increased expression of IL-1beta converting enzyme in hippocampus after ischemia: selective localization in microglia. J Neurosci. 1996 Jul 1;16(13):4146–4154. doi: 10.1523/JNEUROSCI.16-13-04146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchkarev V. A., Eichmüller S., Peters E. M., Pietsch P., Johansson O., Maurer M., Paus R. A simple immunofluorescence technique for simultaneous visualization of mast cells and nerve fibers reveals selectivity and hair cycle--dependent changes in mast cell--nerve fiber contacts in murine skin. Arch Dermatol Res. 1997 Apr;289(5):292–302. doi: 10.1007/s004030050195. [DOI] [PubMed] [Google Scholar]
- Bothwell M. p75NTR: a receptor after all. Science. 1996 Apr 26;272(5261):506–507. doi: 10.1126/science.272.5261.506. [DOI] [PubMed] [Google Scholar]
- CHASE H. B. Growth of the hair. Physiol Rev. 1954 Jan;34(1):113–126. doi: 10.1152/physrev.1954.34.1.113. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P., Carter B. D., Dobrowsky R. T., Chao M. V. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996 Oct 24;383(6602):716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Cece R., Cazzaniga S., Morelli D., Sfondrini L., Bignotto M., Ménard S., Colnaghi M. I., Balsari A. Apoptosis of hair follicle cells during doxorubicin-induced alopecia in rats. Lab Invest. 1996 Oct;75(4):601–609. [PubMed] [Google Scholar]
- Chinnaiyan A. M., Orth K., O'Rourke K., Duan H., Poirier G. G., Dixit V. M. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996 Mar 1;271(9):4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A. M., Tepper C. G., Seldin M. F., O'Rourke K., Kischkel F. C., Hellbardt S., Krammer P. H., Peter M. E., Dixit V. M. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem. 1996 Mar 1;271(9):4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Ihle J. N. Contenders in FasL/TNF death signaling. Cell. 1995 May 19;81(4):479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Cosulich S. C., Green S., Clarke P. R. Bcl-2 regulates activation of apoptotic proteases in a cell-free system. Curr Biol. 1996 Aug 1;6(8):997–1005. doi: 10.1016/s0960-9822(02)00644-9. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Sun T. T., Lavker R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990 Jun 29;61(7):1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Eichmüller S., Stevenson P. A., Paus R. A new method for double immunolabelling with primary antibodies from identical species. J Immunol Methods. 1996 Apr 19;190(2):255–265. doi: 10.1016/0022-1759(95)00281-2. [DOI] [PubMed] [Google Scholar]
- Enari M., Talanian R. V., Wong W. W., Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996 Apr 25;380(6576):723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Novick D., Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990 Jan 25;265(3):1531–1536. [PubMed] [Google Scholar]
- Fearnhead H. O., MacFarlane M., Dinsdale D., Cohen G. M. DNA degradation and proteolysis in thymocyte apoptosis. Toxicol Lett. 1995 Dec;82-83:135–141. doi: 10.1016/0378-4274(95)03473-0. [DOI] [PubMed] [Google Scholar]
- Frade J. M., Rodríguez-Tébar A., Barde Y. A. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996 Sep 12;383(6596):166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Fraser A., Evan G. A license to kill. Cell. 1996 Jun 14;85(6):781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L., Potten C. S. Changes after irradiation in the number of mitotic cells and apoptotic fragments in growing mouse hair follicles and in the width of their hairs. Radiat Res. 1990 Jul;123(1):75–81. [PubMed] [Google Scholar]
- Giannakis C., Forbes I. J., Zalewski P. D. Ca2+/Mg(2+)-dependent nuclease: tissue distribution, relationship to inter-nucleosomal DNA fragmentation and inhibition by Zn2+. Biochem Biophys Res Commun. 1991 Dec 16;181(2):915–920. doi: 10.1016/0006-291x(91)91278-k. [DOI] [PubMed] [Google Scholar]
- Goldberg M. T., Tackaberry L. E., Hardy M. H., Noseworthy J. H. Nuclear aberrations in hair follicle cells of patients receiving cyclophosphamide. A possible in vivo assay for human exposure to genotoxic agents. Arch Toxicol. 1990;64(2):116–121. doi: 10.1007/BF01974396. [DOI] [PubMed] [Google Scholar]
- Golstein P. Controlling cell death. Science. 1997 Feb 21;275(5303):1081–1082. doi: 10.1126/science.275.5303.1081. [DOI] [PubMed] [Google Scholar]
- Gong J., Traganos F., Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994 May 1;218(2):314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- Handjiski B. K., Eichmüller S., Hofmann U., Czarnetzki B. M., Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994 Sep;131(3):303–310. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- Hardy M. H. The secret life of the hair follicle. Trends Genet. 1992 Feb;8(2):55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M. bcl-2 in cancer, development and apoptosis. J Cell Sci Suppl. 1994;18:51–55. doi: 10.1242/jcs.1994.supplement_18.7. [DOI] [PubMed] [Google Scholar]
- Hollis D. E., Chapman R. E. Apoptosis in wool follicles during mouse epidermal growth factor (mEGF)-induced catagen regression. J Invest Dermatol. 1987 Apr;88(4):455–458. doi: 10.1111/1523-1747.ep12469872. [DOI] [PubMed] [Google Scholar]
- Huang B., Eberstadt M., Olejniczak E. T., Meadows R. P., Fesik S. W. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature. 1996 Dec 19;384(6610):638–641. doi: 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- Hug P., Sleight R. G. Liposomes for the transformation of eukaryotic cells. Biochim Biophys Acta. 1991 Jul 26;1097(1):1–17. doi: 10.1016/0925-4439(91)90016-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Proteolytic cleavage during chemotherapy-induced apoptosis. Mol Med Today. 1996 Jul;2(7):298–303. doi: 10.1016/1357-4310(96)10023-x. [DOI] [PubMed] [Google Scholar]
- Kitson J., Raven T., Jiang Y. P., Goeddel D. V., Giles K. M., Pun K. T., Grinham C. J., Brown R., Farrow S. N. A death-domain-containing receptor that mediates apoptosis. Nature. 1996 Nov 28;384(6607):372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- Krajewski S., Krajewska M., Shabaik A., Miyashita T., Wang H. G., Reed J. C. Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol. 1994 Dec;145(6):1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Petit P., Zamzami N., Vayssière J. L., Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995 Oct;9(13):1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Zamzami N., Susin S. A. Mitochondrial control of apoptosis. Immunol Today. 1997 Jan;18(1):44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- Lasic D. D., Papahadjopoulos D. Liposomes revisited. Science. 1995 Mar 3;267(5202):1275–1276. doi: 10.1126/science.7871422. [DOI] [PubMed] [Google Scholar]
- Li L., Hoffman R. M. The feasibility of targeted selective gene therapy of the hair follicle. Nat Med. 1995 Jul;1(7):705–706. doi: 10.1038/nm0795-705. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Wrone-Smith T., Simonian P., Foreman K. E., Nunez G., Nickoloff B. J. Apoptosis in keratinocytes is not dependent on induction of differentiation. Lab Invest. 1997 Jan;76(1):99–107. [PubMed] [Google Scholar]
- Moore G. P., Panaretto B. A., Carter N. B. Epidermal hyperplasia and wool follicle regression in sheep infused with epidermal growth factor. J Invest Dermatol. 1985 Mar;84(3):172–175. doi: 10.1111/1523-1747.ep12264699. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997 Feb 7;88(3):355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Ogasawara J., Suda T., Nagata S. Selective apoptosis of CD4+CD8+ thymocytes by the anti-Fas antibody. J Exp Med. 1995 Feb 1;181(2):485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Parakkal P. F. Ultrastructural changes of the basal lamina during the hair growth cycle. J Cell Biol. 1969 Feb;40(2):561–564. doi: 10.1083/jcb.40.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R., Handjiski B., Czarnetzki B. M., Eichmüller S. A murine model for inducing and manipulating hair follicle regression (catagen): effects of dexamethasone and cyclosporin A. J Invest Dermatol. 1994 Aug;103(2):143–147. doi: 10.1111/1523-1747.ep12392542. [DOI] [PubMed] [Google Scholar]
- Paus R., Handjiski B., Eichmüller S., Czarnetzki B. M. Chemotherapy-induced alopecia in mice. Induction by cyclophosphamide, inhibition by cyclosporine A, and modulation by dexamethasone. Am J Pathol. 1994 Apr;144(4):719–734. [PMC free article] [PubMed] [Google Scholar]
- Paus R., Hofmann U., Eichmüller S., Czarnetzki B. M. Distribution and changing density of gamma-delta T cells in murine skin during the induced hair cycle. Br J Dermatol. 1994 Mar;130(3):281–289. doi: 10.1111/j.1365-2133.1994.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Paus R., Menrad A., Czarnetzki B. M. Nekrobiologie der Haut: Apoptose. Hautarzt. 1995 Apr;46(4):285–303. [PubMed] [Google Scholar]
- Paus R., Rosenbach T., Haas N., Czarnetzki B. M. Patterns of cell death: the significance of apoptosis for dermatology. Exp Dermatol. 1993 Feb;2(1):3–11. doi: 10.1111/j.1600-0625.1993.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Paus R., Schilli M. B., Handjiski B., Menrad A., Henz B. M., Plonka P. Topical calcitriol enhances normal hair regrowth but does not prevent chemotherapy-induced alopecia in mice. Cancer Res. 1996 Oct 1;56(19):4438–4443. [PubMed] [Google Scholar]
- Paus R., Stenn K. S., Link R. E. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990 Jun;122(6):777–784. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- Pierard-Franchimont C., Pierard G. E. Massive lymphocyte-mediated apoptosis during the early stage of pseudopelade. Dermatologica. 1986;172(5):254–257. doi: 10.1159/000249350. [DOI] [PubMed] [Google Scholar]
- Polakowska R. R., Haake A. R. Apoptosis: the skin from a new perspective. Cell Death Differ. 1994 Jul;1(1):19–31. [PubMed] [Google Scholar]
- Polakowska R. R., Piacentini M., Bartlett R., Goldsmith L. A., Haake A. R. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994 Mar;199(3):176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- Potten C. S. Cell death (apoptosis) in hair follicles and consequent changes in the width of hairs after irradiation of growing follicles. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Sep;48(3):349–360. doi: 10.1080/09553008514551351. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993 Oct 29;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- STRAILE W. E., CHASE H. B., ARSENAULT C. Growth and differentiation of hair follicles between periods of activity and quiescence. J Exp Zool. 1961 Dec;148:205–221. doi: 10.1002/jez.1401480304. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Milligan C. E. Cold thoughts of death: the role of ICE proteases in neuronal cell death. Trends Neurosci. 1996 Dec;19(12):555–562. doi: 10.1016/s0166-2236(96)10067-9. [DOI] [PubMed] [Google Scholar]
- Schwarz A., Bhardwaj R., Aragane Y., Mahnke K., Riemann H., Metze D., Luger T. A., Schwarz T. Ultraviolet-B-induced apoptosis of keratinocytes: evidence for partial involvement of tumor necrosis factor-alpha in the formation of sunburn cells. J Invest Dermatol. 1995 Jun;104(6):922–927. doi: 10.1111/1523-1747.ep12606202. [DOI] [PubMed] [Google Scholar]
- Seiberg M., Marthinuss J. Clusterin expression within skin correlates with hair growth. Dev Dyn. 1995 Mar;202(3):294–301. doi: 10.1002/aja.1002020308. [DOI] [PubMed] [Google Scholar]
- Seiberg M., Marthinuss J., Stenn K. S. Changes in expression of apoptosis-associated genes in skin mark early catagen. J Invest Dermatol. 1995 Jan;104(1):78–82. doi: 10.1111/1523-1747.ep12613555. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kamiike W., Matsuda H., Tsujimoto Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene. 1996 Jun 6;12(11):2251–2257. [PubMed] [Google Scholar]
- Slominski A., Paus R., Plonka P., Chakraborty A., Maurer M., Pruski D., Lukiewicz S. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J Invest Dermatol. 1994 Jun;102(6):862–869. doi: 10.1111/1523-1747.ep12382606. [DOI] [PubMed] [Google Scholar]
- Slominski A., Paus R., Plonka P., Handjiski B., Maurer M., Chakraborty A., Mihm M. C., Jr Pharmacological disruption of hair follicle pigmentation by cyclophosphamide as a model for studying the melanocyte response to and recovery from cytotoxic drug damage in situ. J Invest Dermatol. 1996 Jun;106(6):1203–1211. doi: 10.1111/1523-1747.ep12348479. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Farrah T., Goodwin R. G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994 Mar 25;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Stenn K. S., Combates N. J., Eilertsen K. J., Gordon J. S., Pardinas J. R., Parimoo S., Prouty S. M. Hair follicle growth controls. Dermatol Clin. 1996 Oct;14(4):543–558. doi: 10.1016/s0733-8635(05)70383-1. [DOI] [PubMed] [Google Scholar]
- Stenn K. S., Lawrence L., Veis D., Korsmeyer S., Seiberg M. Expression of the bcl-2 protooncogene in the cycling adult mouse hair follicle. J Invest Dermatol. 1994 Jul;103(1):107–111. doi: 10.1111/1523-1747.ep12391844. [DOI] [PubMed] [Google Scholar]
- Veis D. J., Sorenson C. M., Shutter J. R., Korsmeyer S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993 Oct 22;75(2):229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Weedon D., Strutton G. Apoptosis as the mechanism of the involution of hair follicles in catagen transformation. Acta Derm Venereol. 1981;61(4):335–339. [PubMed] [Google Scholar]
- Welker P., Foitzik K., Bulfone-Paus S., Henz B. M., Paus R. Hair cycle-dependent changes in the gene expression and protein content of transforming factor beta 1 and beta 3 in murine skin. Arch Dermatol Res. 1997 Aug;289(9):554–557. doi: 10.1007/s004030050239. [DOI] [PubMed] [Google Scholar]
- White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996 Jan 1;10(1):1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B., Gahm A., Ankarcrona M., Nicotera P., Orrenius S. Multiple proteases are involved in thymocyte apoptosis. Exp Cell Res. 1995 Dec;221(2):404–412. doi: 10.1006/excr.1995.1391. [DOI] [PubMed] [Google Scholar]