Abstract
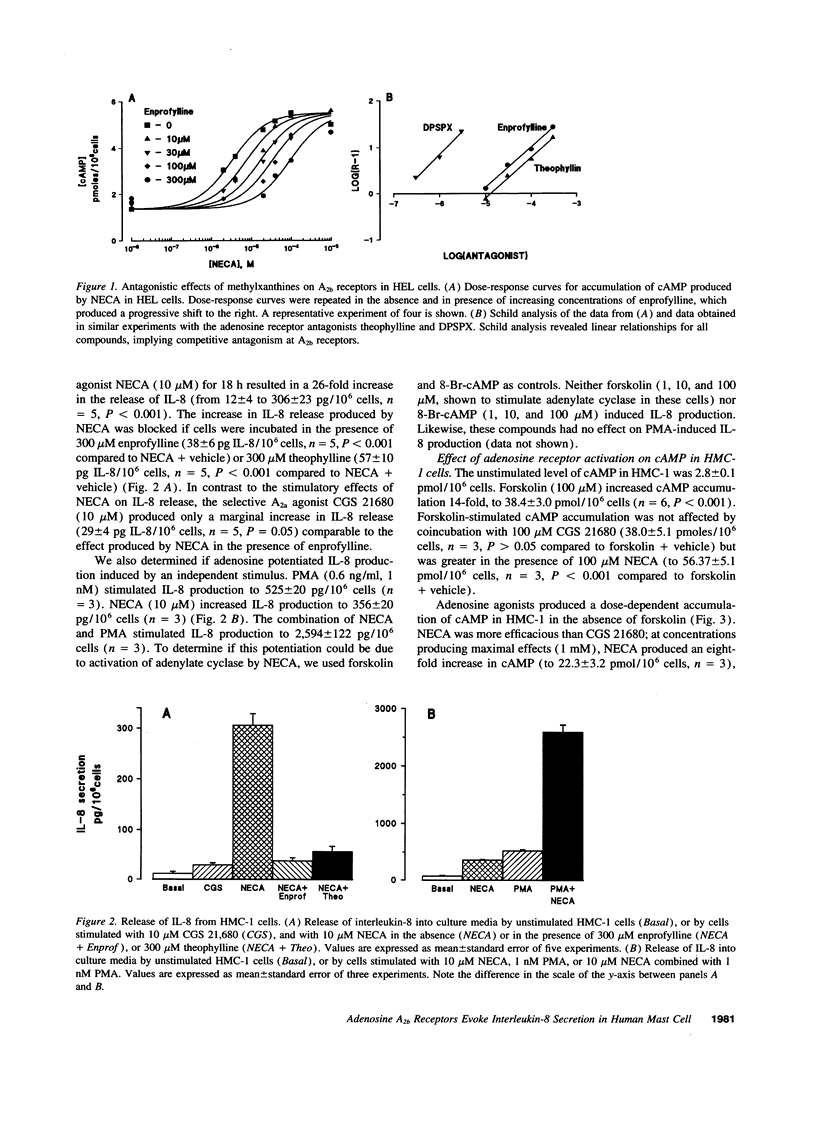
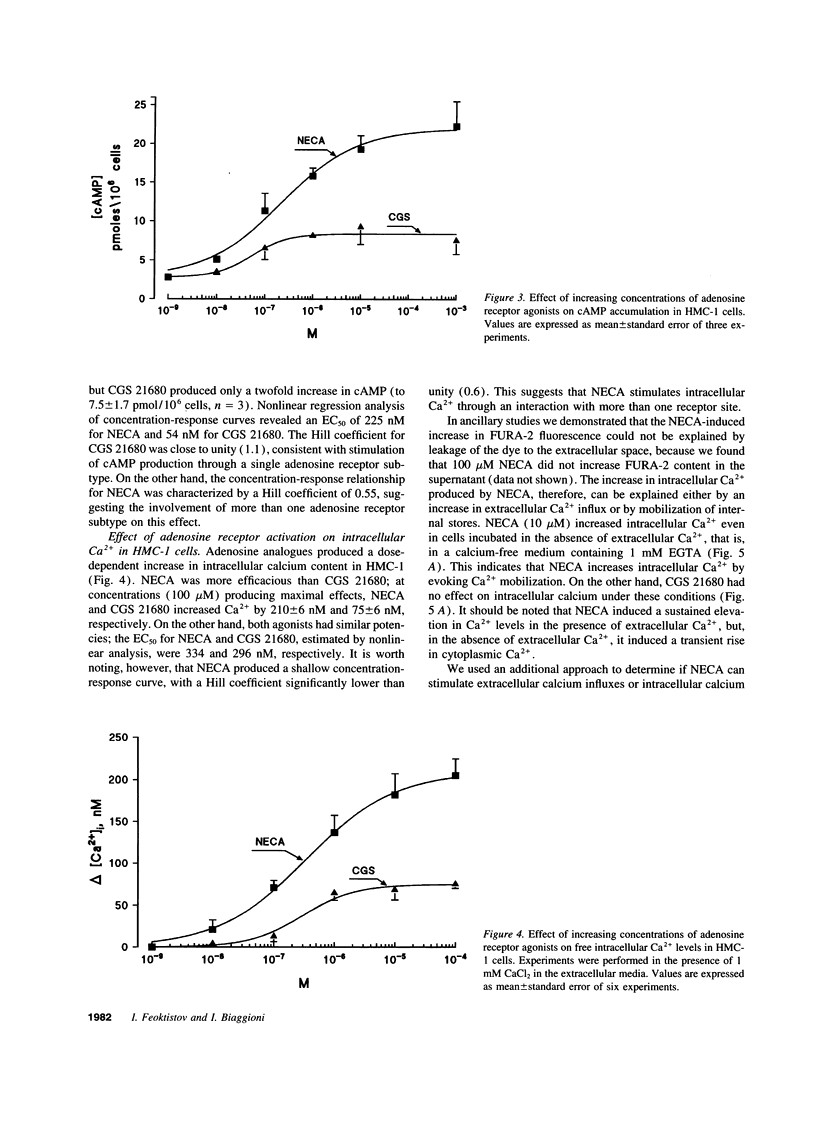
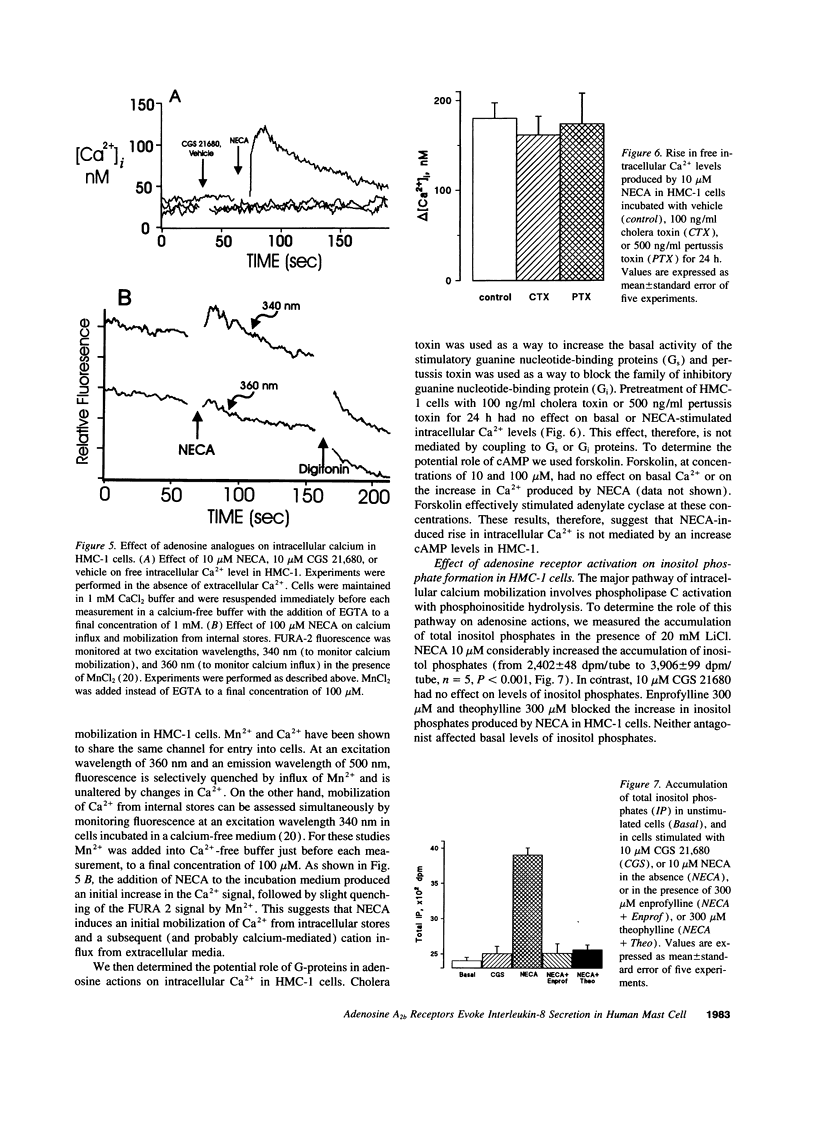
Adenosine potentiates mast cell activation, but the receptor type and molecular mechanisms involved have not been defined. We, therefore, investigated the effects of adenosine on the human mast cell line HMC-1. Both the A2a selective agonist CGS21680 and the A2a/A2b nonselective agonist 5'-N-ethylcarboxamidoadenosine (NECA) increased cAMP, but NECA was fourfold more efficacious and had a Hill coefficient of 0.55, suggesting the presence of both A2a and A2b receptors. NECA 10 microM evoked IL-8 release from HMC-1, but CGS21680 10 microM had no effect. In separate studies we found that enprofylline, an antiasthmatic previously thought to lack adenosine antagonistic properties, is as effective as theophylline as an antagonist of A2b receptors at concentrations achieved clinically. Both theophylline and enprofylline 300 micro completely blocked the release of IL-8 by NECA. NECA, but not CGS21680, increases inositol phosphate formation and intracellular calcium mobilization through a cholera and pertussis toxin-insensitive mechanism. In conclusion, both A2a and A2b receptors are present in HMC-1 cells and are coupled to adenylate cyclase. In addition, A2b receptors are coupled to phospholipase C and evoke IL-8 release. This effect is blocked by theophylline and enprofylline, raising the possibility that this mechanism contributes to their antiasthmatic effects.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Beaven M. A., Ramkumar V., Ali H. Adenosine A3 receptors in mast cells. Trends Pharmacol Sci. 1994 Jan;15(1):13–14. doi: 10.1016/0165-6147(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Biaggioni I., Paul S., Puckett A., Arzubiaga C. Caffeine and theophylline as adenosine receptor antagonists in humans. J Pharmacol Exp Ther. 1991 Aug;258(2):588–593. [PubMed] [Google Scholar]
- Björck T., Gustafsson L. E., Dahlén S. E. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis. 1992 May;145(5):1087–1091. doi: 10.1164/ajrccm/145.5.1087. [DOI] [PubMed] [Google Scholar]
- Bradley A. B., Morgan K. G. Cellular Ca2+ monitored by aequorin in adenosine-mediated smooth muscle relaxation. Am J Physiol. 1985 Jan;248(1 Pt 2):H109–H117. doi: 10.1152/ajpheart.1985.248.1.H109. [DOI] [PubMed] [Google Scholar]
- Butterfield J. H., Weiler D., Dewald G., Gleich G. J. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Church M. K., Hughes P. J., Vardey C. J. Studies on the receptor mediating cyclic AMP-independent enhancement by adenosine of IgE-dependent mediator release from rat mast cells. Br J Pharmacol. 1986 Jan;87(1):233–242. doi: 10.1111/j.1476-5381.1986.tb10176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H., Cushley M. J., Persson C. G., Holgate S. T. The protective effects of intravenous theophylline and enprofylline against histamine- and adenosine 5'-monophosphate-provoked bronchoconstriction: implications for the mechanisms of action of xanthine derivatives in asthma. Pulm Pharmacol. 1989;2(3):147–154. doi: 10.1016/0952-0600(89)90039-2. [DOI] [PubMed] [Google Scholar]
- Crimi E., Brusasco V., Brancatisano M., Losurdo E., Crimi P. Adenosine-induced bronchoconstriction: premedication with chlorpheniramine and nedocromil sodium. Eur J Respir Dis Suppl. 1986;147:255–257. [PubMed] [Google Scholar]
- Cushley M. J., Holgate S. T. Adenosine-induced bronchoconstriction in asthma: role of mast cell-mediator release. J Allergy Clin Immunol. 1985 Feb;75(2):272–278. doi: 10.1016/0091-6749(85)90057-0. [DOI] [PubMed] [Google Scholar]
- Cushley M. J., Tattersfield A. E., Holgate S. T. Adenosine-induced bronchoconstriction in asthma. Antagonism by inhaled theophylline. Am Rev Respir Dis. 1984 Mar;129(3):380–384. doi: 10.1164/arrd.1984.129.3.380. [DOI] [PubMed] [Google Scholar]
- Feoktistov I. A., Paul S., Hollister A. S., Robertson D., Biaggioni I. Role of cyclic AMP in adenosine inhibition of intracellular calcium rise in human platelets. Comparison of adenosine effects on thrombin- and epinephrine-induced platelet stimulation. Am J Hypertens. 1992 Jun;5(6 Pt 2):147S–153S. doi: 10.1093/ajh/5.6.147s. [DOI] [PubMed] [Google Scholar]
- Feoktistov I., Biaggioni I. Characterization of adenosine receptors in human erythroleukemia cells and platelets: further evidence for heterogeneity of adenosine A2 receptor subtypes. Mol Pharmacol. 1993 Jun;43(6):909–914. [PubMed] [Google Scholar]
- Feoktistov I., Murray J. J., Biaggioni I. Positive modulation of intracellular Ca2+ levels by adenosine A2b receptors, prostacyclin, and prostaglandin E1 via a cholera toxin-sensitive mechanism in human erythroleukemia cells. Mol Pharmacol. 1994 Jun;45(6):1160–1167. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamilton S. L., Codina J., Hawkes M. J., Yatani A., Sawada T., Strickland F. M., Froehner S. C., Spiegel A. M., Toro L., Stefani E. Evidence for direct interaction of Gs alpha with the Ca2+ channel of skeletal muscle. J Biol Chem. 1991 Oct 15;266(29):19528–19535. [PubMed] [Google Scholar]
- Hillyard P. A., Nials A. T., Skidmore I. F., Vardey C. J. Characterization of the adenosine receptor responsible for the inhibition of histamine and SRS-A release from human lung fragments. Br J Pharmacol. 1984 Oct;83(2):337–345. doi: 10.1111/j.1476-5381.1984.tb16493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Lewis R. A., Austen K. F. Role of adenylate cyclase in immunologic release of mediators from rat mast cells: agonist and antagonist effects of purine- and ribose-modified adenosine analogs. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6800–6804. doi: 10.1073/pnas.77.11.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. J., Holgate S. T., Church M. K. Adenosine inhibits and potentiates IgE-dependent histamine release from human lung mast cells by an A2-purinoceptor mediated mechanism. Biochem Pharmacol. 1984 Dec 1;33(23):3847–3852. doi: 10.1016/0006-2952(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J., Taylor H. E., Robeva A. S., Tucker A. L., Stehle J. H., Rivkees S. A., Fink J. S., Reppert S. M. Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol. 1993 Sep;44(3):524–532. [PubMed] [Google Scholar]
- Mann J. S., Holgate S. T. Specific antagonism of adenosine-induced bronchoconstriction in asthma by oral theophylline. Br J Clin Pharmacol. 1985 May;19(5):685–692. doi: 10.1111/j.1365-2125.1985.tb02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt D. L., Parker C. W., Sullivan T. J. Potentiation of mast cell mediator release by adenosine. J Immunol. 1978 Mar;120(3):871–878. [PubMed] [Google Scholar]
- Marquardt D. L., Walker L. L. Alteration of mast cell responsiveness to adenosine by pertussis toxin. Biochem Pharmacol. 1988 Oct 15;37(20):4019–4025. doi: 10.1016/0006-2952(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Marquardt D. L., Walker L. L., Heinemann S. Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol. 1994 May 1;152(9):4508–4515. [PubMed] [Google Scholar]
- Marquardt D. L., Walker L. L. Modulation of mast cell responses to adenosine by agents that alter protein kinase C activity. Biochem Pharmacol. 1990 Jun 15;39(12):1929–1934. doi: 10.1016/0006-2952(90)90611-n. [DOI] [PubMed] [Google Scholar]
- Marquardt D. L., Walker L. L., Wasserman S. I. Adenosine receptors on mouse bone marrow-derived mast cells: functional significance and regulation by aminophylline. J Immunol. 1984 Aug;133(2):932–937. [PubMed] [Google Scholar]
- Merritt J. E., Jacob R., Hallam T. J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem. 1989 Jan 25;264(3):1522–1527. [PubMed] [Google Scholar]
- Mogul D. J., Adams M. E., Fox A. P. Differential activation of adenosine receptors decreases N-type but potentiates P-type Ca2+ current in hippocampal CA3 neurons. Neuron. 1993 Feb;10(2):327–334. doi: 10.1016/0896-6273(93)90322-i. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Blom T., Kusche-Gullberg M., Kjellén L., Butterfield J. H., Sundström C., Nilsson K., Hellman L. Phenotypic characterization of the human mast-cell line HMC-1. Scand J Immunol. 1994 May;39(5):489–498. doi: 10.1111/j.1365-3083.1994.tb03404.x. [DOI] [PubMed] [Google Scholar]
- Peachell P. T., Lichtenstein L. M., Schleimer R. P. Differential regulation of human basophil and lung mast cell function by adenosine. J Pharmacol Exp Ther. 1991 Feb;256(2):717–726. [PubMed] [Google Scholar]
- Persson C. G., Andersson K. E., Kjellin G. Effects of enprofylline and theophylline may show the role of adenosine. Life Sci. 1986 Mar 24;38(12):1057–1072. doi: 10.1016/0024-3205(86)90241-9. [DOI] [PubMed] [Google Scholar]
- Persson C. G. The profile of action of enprofylline, or why adenosine antagonism seems less desirable with xanthine antiasthmatics. Agents Actions Suppl. 1983;13:115–129. [PubMed] [Google Scholar]
- Rafferty P., Beasley R., Holgate S. T. The contribution of histamine to immediate bronchoconstriction provoked by inhaled allergen and adenosine 5' monophosphate in atopic asthma. Am Rev Respir Dis. 1987 Aug;136(2):369–373. doi: 10.1164/ajrccm/136.2.369. [DOI] [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L., Beaven M. A., Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993 Aug 15;268(23):16887–16890. [PubMed] [Google Scholar]
- Salvatore C. A., Jacobson M. A., Taylor H. E., Linden J., Johnson R. G. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scamps F., Rybin V., Puceat M., Tkachuk V., Vassort G. A Gs protein couples P2-purinergic stimulation to cardiac Ca channels without cyclic AMP production. J Gen Physiol. 1992 Oct;100(4):675–701. doi: 10.1085/jgp.100.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvan R. S., Butterfield J. H., Krangel M. S. Expression of multiple chemokine genes by a human mast cell leukemia. J Biol Chem. 1994 May 13;269(19):13893–13898. [PubMed] [Google Scholar]
- Zhou Q. Y., Li C., Olah M. E., Johnson R. A., Stiles G. L., Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]



