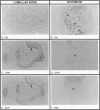
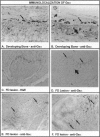
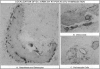
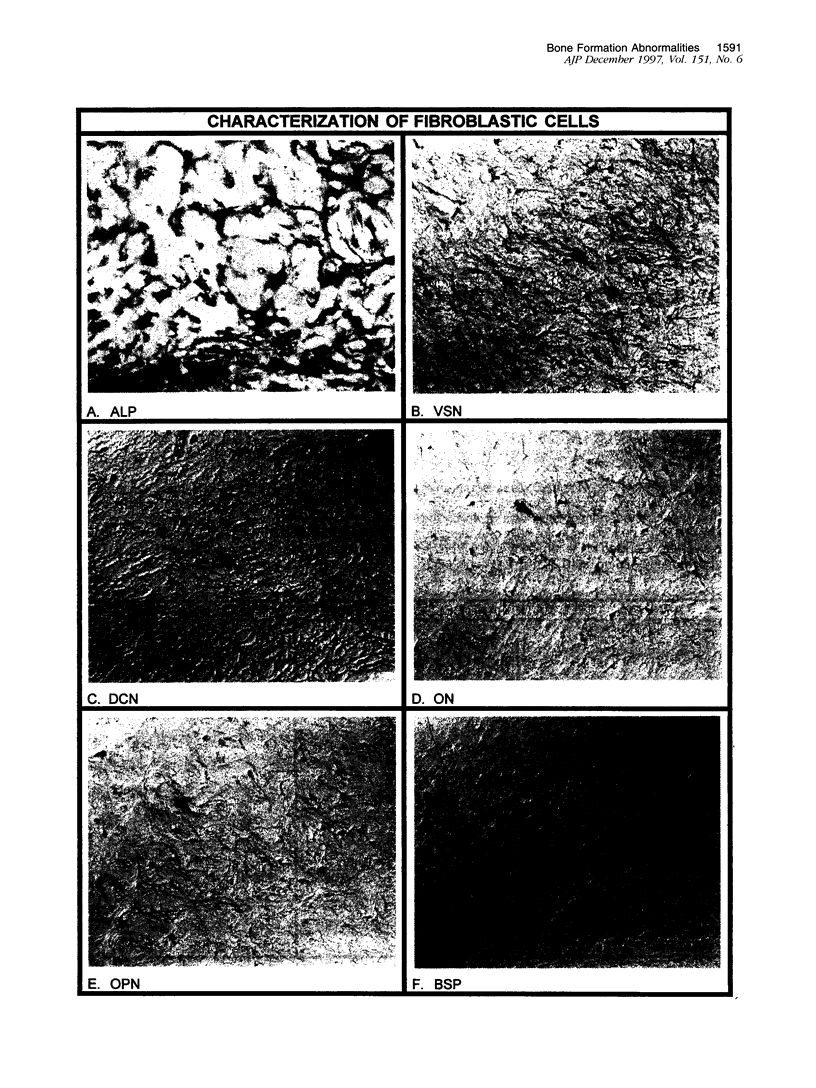
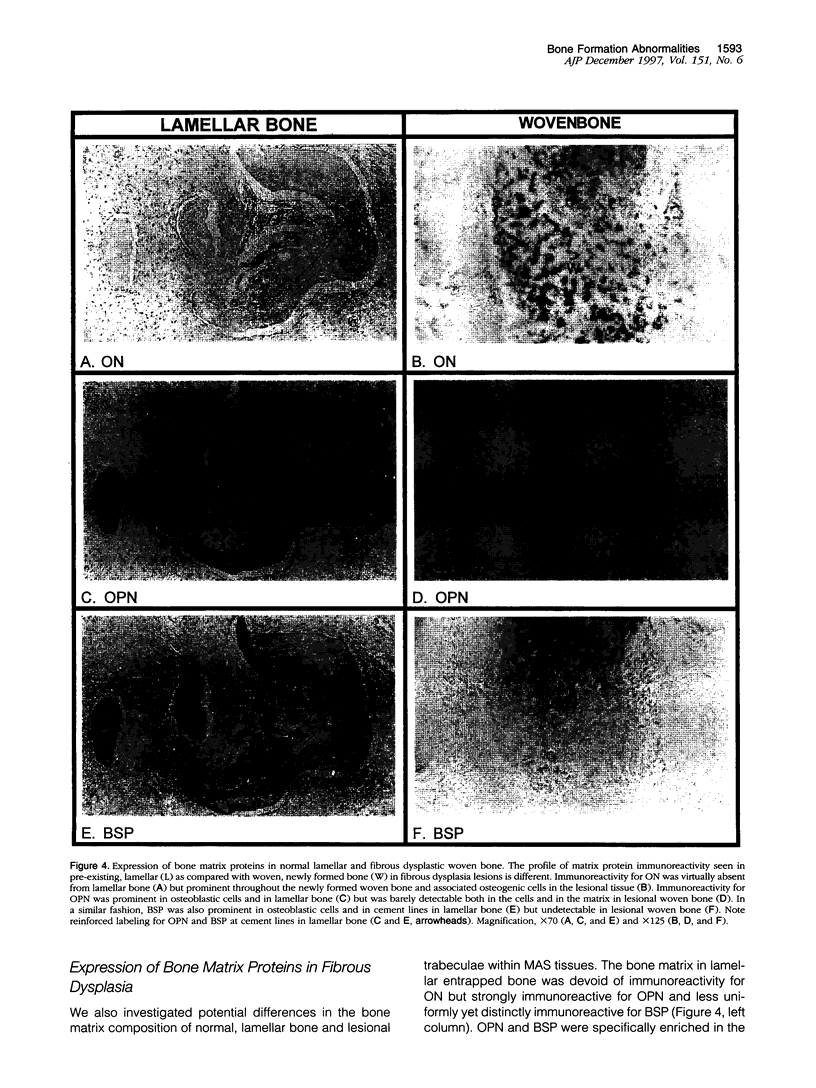
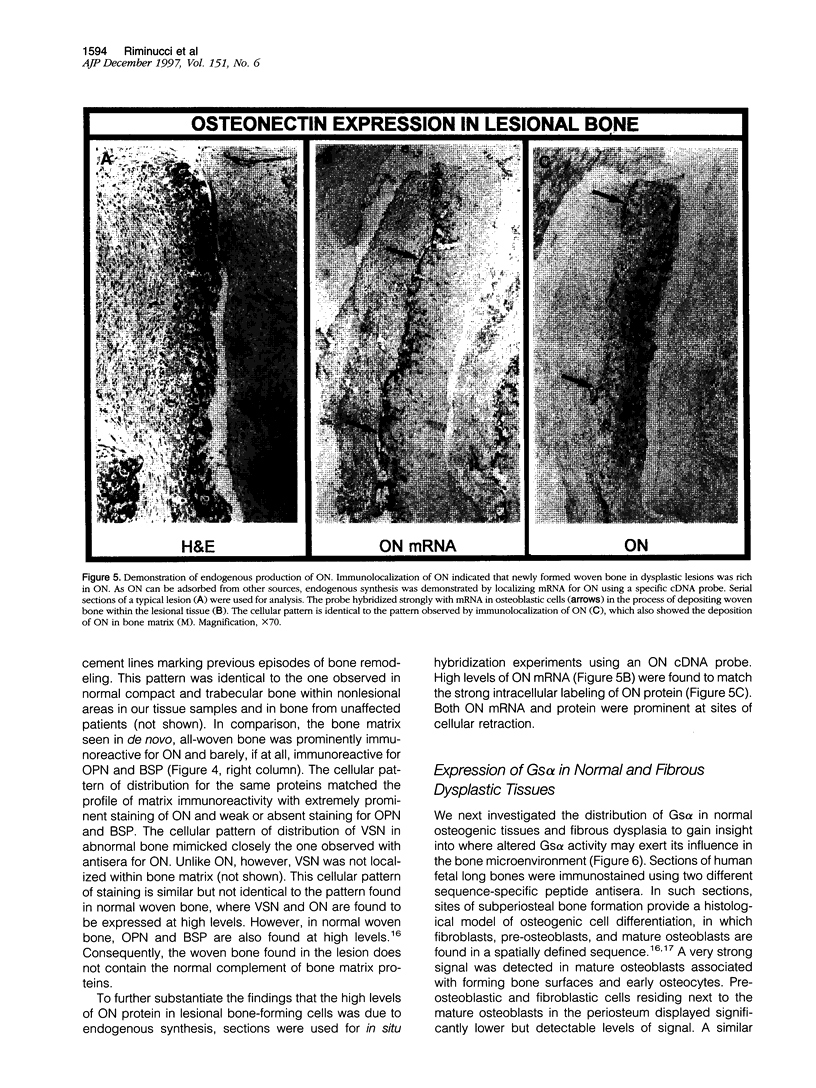
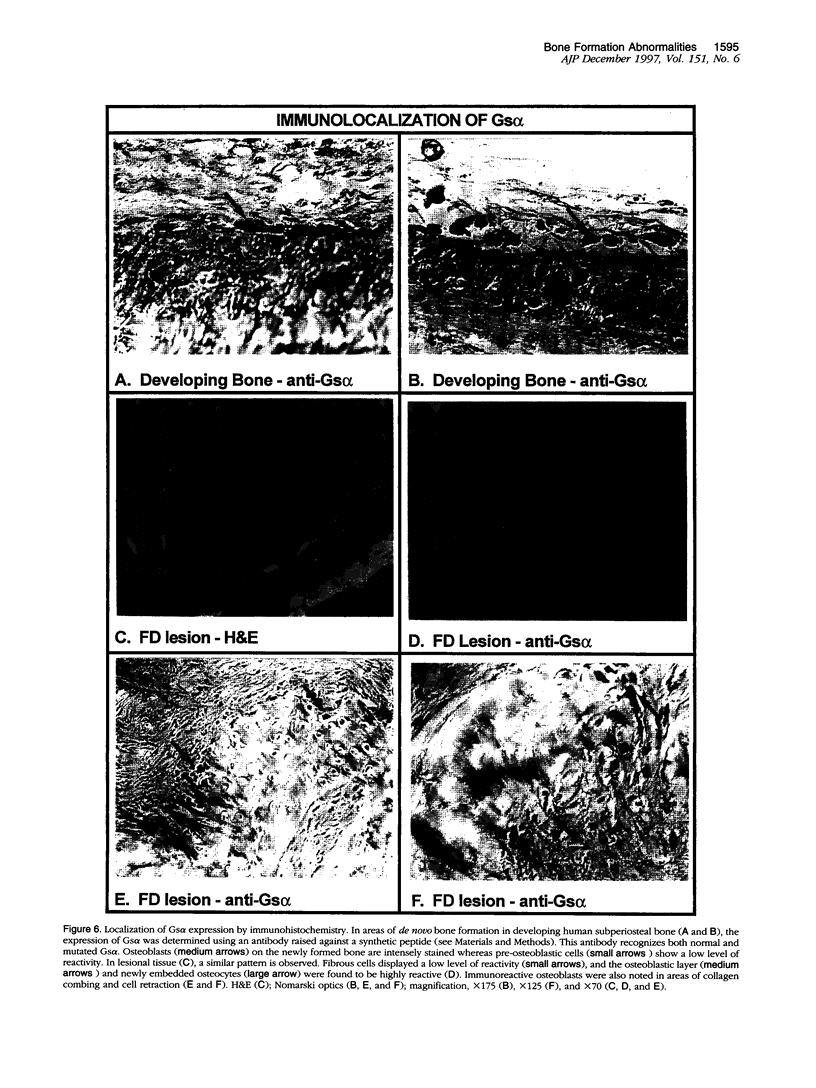
Abstract
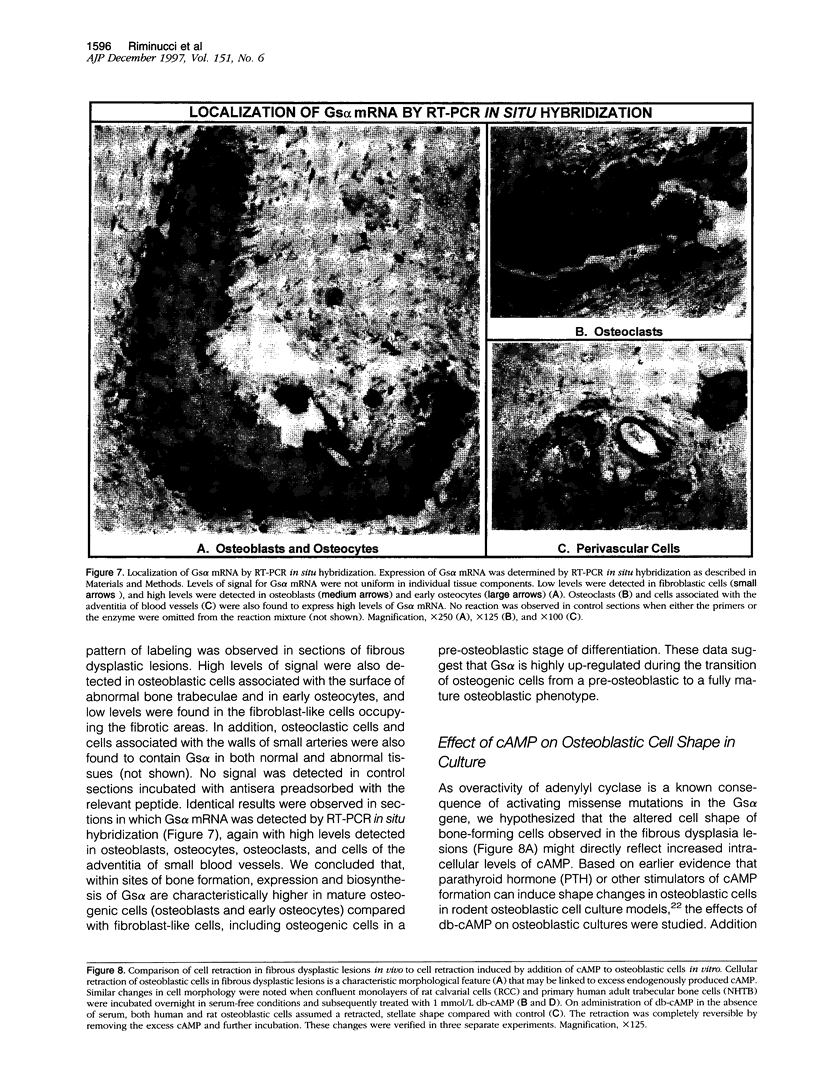
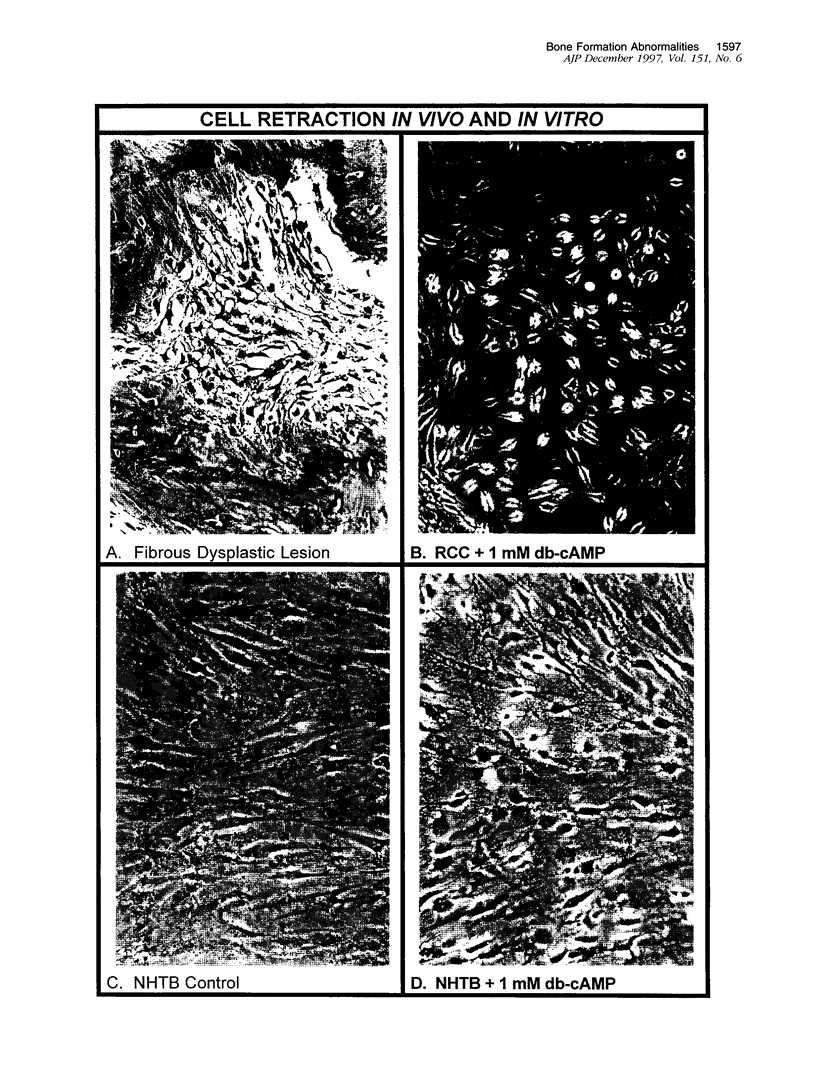
In addition to café-au-lait pigmentation patterns and hyperendocrinopathies, fibrous dysplasia of bone is a major finding in the McCune-Albright syndrome. Activating missense mutations of the Gs alpha gene leading to overactivity of adenylyl cyclase have been identified in patients with McCune-Albright syndrome, but the mechanism leading to the specific development of fibrous dysplasia in bone has not been elucidated. By means of specific peptide antisera and reverse transcriptase polymerase chain reaction in situ hybridization, we show that expression of Gs alpha and its mRNA is critically up-regulated during maturation of precursor osteogenic cells to normal osteoblast cells and that this pattern of expression is retained in fibrous dysplasia. A functional characterization of fibrous dysplastic tissues revealed that the fibrotic areas consist, in fact, of an excess of cells with phenotypic features of pre-osteogenic cells, whereas the lesional bone formed de novo within fibrotic areas represents the biosynthetic output of mature but abnormal osteoblasts. These cells are noted for peculiar changes in cell shape and interaction with matrix, which were mimicked in vitro by the effects of excess exogenous cAMP on human osteogenic cells. Osteoblasts involved with the de novo deposition of lesional bone in fibrous dysplasia produce a bone matrix enriched in certain anti-adhesion molecules (versican and osteonectin), and poor in the pro-adhesive molecules osteopontin and bone sialoprotein, which is in contrast to the high levels of these two proteins found in normal de novo bone. Our data indicate the need to reinterpret fibrous dysplasia of bone as a disease of cells in the osteogenic lineage, related to the effects of excess cAMP on bone cell function. They further suggest that a critical, physiological, maturation-related regulation of Gs alpha levels makes cells in the osteogenic lineage a natural target for the effects of mutations in the Gs alpha gene and may provide a clue as to why bone itself is affected in this somatic, mutation-dependent disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alman B. A., Greel D. A., Wolfe H. J. Activating mutations of Gs protein in monostotic fibrous lesions of bone. J Orthop Res. 1996 Mar;14(2):311–315. doi: 10.1002/jor.1100140221. [DOI] [PubMed] [Google Scholar]
- Bianco P., Bonucci E. Endosteal surfaces in hyperparathyroidism: an enzyme cytochemical study on low-temperature-processed, glycol-methacrylate-embedded bone biopsies. Virchows Arch A Pathol Anat Histopathol. 1991;419(5):425–431. doi: 10.1007/BF01605077. [DOI] [PubMed] [Google Scholar]
- Bianco P., Boyde A. Alkaline phosphatase cytochemistry in confocal scanning light microscopy for imaging the bone marrow stroma. Basic Appl Histochem. 1989;33(1):17–23. [PubMed] [Google Scholar]
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J Histochem Cytochem. 1990 Nov;38(11):1549–1563. doi: 10.1177/38.11.2212616. [DOI] [PubMed] [Google Scholar]
- Bianco P., Fisher L. W., Young M. F., Termine J. D., Robey P. G. Expression of bone sialoprotein (BSP) in developing human tissues. Calcif Tissue Int. 1991 Dec;49(6):421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- Bianco P., Riminucci M., Bonucci E., Termine J. D., Robey P. G. Bone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J Histochem Cytochem. 1993 Feb;41(2):183–191. doi: 10.1177/41.2.8419458. [DOI] [PubMed] [Google Scholar]
- Bianco P., Silvestrini G., Termine J. D., Bonucci E. Immunohistochemical localization of osteonectin in developing human and calf bone using monoclonal antibodies. Calcif Tissue Int. 1988 Sep;43(3):155–161. doi: 10.1007/BF02571313. [DOI] [PubMed] [Google Scholar]
- Candeliere G. A., Glorieux F. H., Prud'homme J., St-Arnaud R. Increased expression of the c-fos proto-oncogene in bone from patients with fibrous dysplasia. N Engl J Med. 1995 Jun 8;332(23):1546–1551. doi: 10.1056/NEJM199506083322304. [DOI] [PubMed] [Google Scholar]
- Danon M., Crawford J. D. The McCune-Albright syndrome. Ergeb Inn Med Kinderheilkd. 1987;55:81–115. doi: 10.1007/978-3-642-71052-0_3. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Lopez C. A., Rollo E. E., Hwang S. M., An X. R., Walther S. E. Osteopontin-induced modifications of cellular functions. Ann N Y Acad Sci. 1995 Apr 21;760:127–142. doi: 10.1111/j.1749-6632.1995.tb44625.x. [DOI] [PubMed] [Google Scholar]
- Everitt E. A., Sage E. H. Expression of SPARC is correlated with altered morphologies in transfected F9 embryonal carcinoma cells. Exp Cell Res. 1992 Mar;199(1):134–146. doi: 10.1016/0014-4827(92)90471-j. [DOI] [PubMed] [Google Scholar]
- Fedarko N. S., Termine J. D., Young M. F., Robey P. G. Temporal regulation of hyaluronan and proteoglycan metabolism by human bone cells in vitro. J Biol Chem. 1990 Jul 25;265(21):12200–12209. [PubMed] [Google Scholar]
- Fisher L. W., Stubbs J. T., 3rd, Young M. F. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995 Oct;266:61–65. [PubMed] [Google Scholar]
- Greco M. A., Steiner G. C. Ultrastructure of fibrous dysplasia of bone: a study of its fibrous, osseous, and cartilaginous components. Ultrastruct Pathol. 1986;10(1):55–66. doi: 10.3109/01913128609015563. [DOI] [PubMed] [Google Scholar]
- Kerr J. M., Fisher L. W., Termine J. D., Wang M. G., McBride O. W., Young M. F. The human bone sialoprotein gene (IBSP): genomic localization and characterization. Genomics. 1993 Aug;17(2):408–415. doi: 10.1006/geno.1993.1340. [DOI] [PubMed] [Google Scholar]
- Malchoff C. D., Reardon G., MacGillivray D. C., Yamase H., Rogol A. D., Malchoff D. M. An unusual presentation of McCune-Albright syndrome confirmed by an activating mutation of the Gs alpha-subunit from a bone lesion. J Clin Endocrinol Metab. 1994 Mar;78(3):803–806. doi: 10.1210/jcem.78.3.8126161. [DOI] [PubMed] [Google Scholar]
- Marie P. J., de Pollak C., Chanson P., Lomri A. Increased proliferation of osteoblastic cells expressing the activating Gs alpha mutation in monostotic and polyostotic fibrous dysplasia. Am J Pathol. 1997 Mar;150(3):1059–1069. [PMC free article] [PubMed] [Google Scholar]
- Mark M. P., Butler W. T., Prince C. W., Finkelman R. D., Ruch J. V. Developmental expression of 44-kDa bone phosphoprotein (osteopontin) and bone gamma-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation. 1988;37(2):123–136. doi: 10.1111/j.1432-0436.1988.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Miller S. S., Wolf A. M., Arnaud C. D. Bone cells in culture: morphologic transformation by hormones. Science. 1976 Jun 25;192(4246):1340–1343. doi: 10.1126/science.1273593. [DOI] [PubMed] [Google Scholar]
- Mintz K. P., Grzesik W. J., Midura R. J., Robey P. G., Termine J. D., Fisher L. W. Purification and fragmentation of nondenatured bone sialoprotein: evidence for a cryptic, RGD-resistant cell attachment domain. J Bone Miner Res. 1993 Aug;8(8):985–995. doi: 10.1002/jbmr.5650080812. [DOI] [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteoblast-like cells by parathyroid hormone. J Cell Biol. 1989 Feb;108(2):713–718. doi: 10.1083/jcb.108.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. The origin of bone cells. Int Rev Cytol. 1970;28:213–238. doi: 10.1016/s0074-7696(08)62544-9. [DOI] [PubMed] [Google Scholar]
- Robey P. G., Termine J. D. Human bone cells in vitro. Calcif Tissue Int. 1985 Sep;37(5):453–460. [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Schwindinger W. F., Francomano C. A., Levine M. A. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker A., Chanson P., Weinstein L. S., Chi P., Spiegel A. M., Lomri A., Marie P. J. Osteoblastic cells derived from isolated lesions of fibrous dysplasia contain activating somatic mutations of the Gs alpha gene. Hum Mol Genet. 1995 Sep;4(9):1675–1676. doi: 10.1093/hmg/4.9.1675. [DOI] [PubMed] [Google Scholar]
- Shenker A., Weinstein L. S., Moran A., Pescovitz O. H., Charest N. J., Boney C. M., Van Wyk J. J., Merino M. J., Feuillan P. P., Spiegel A. M. Severe endocrine and nonendocrine manifestations of the McCune-Albright syndrome associated with activating mutations of stimulatory G protein GS. J Pediatr. 1993 Oct;123(4):509–518. doi: 10.1016/s0022-3476(05)80943-6. [DOI] [PubMed] [Google Scholar]
- Shenker A., Weinstein L. S., Sweet D. E., Spiegel A. M. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab. 1994 Sep;79(3):750–755. doi: 10.1210/jcem.79.3.8077356. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Woodard C. J., Unson C. G., Spiegel A. M. Receptor and effector interactions of Gs. Functional studies with antibodies to the alpha s carboxyl-terminal decapeptide. FEBS Lett. 1989 Jun 5;249(2):189–194. doi: 10.1016/0014-5793(89)80622-2. [DOI] [PubMed] [Google Scholar]
- Weinstein L. S., Shenker A., Gejman P. V., Merino M. J., Friedman E., Spiegel A. M. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991 Dec 12;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Ozono K., Kasayama S., Yoh K., Hiroshima K., Takagi M., Matsumoto S., Michigami T., Yamaoka K., Kishimoto T. Increased IL-6-production by cells isolated from the fibrous bone dysplasia tissues in patients with McCune-Albright syndrome. J Clin Invest. 1996 Jul 1;98(1):30–35. doi: 10.1172/JCI118773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. F., Findlay D. M., Dominguez P., Burbelo P. D., McQuillan C., Kopp J. B., Robey P. G., Termine J. D. Osteonectin promoter. DNA sequence analysis and S1 endonuclease site potentially associated with transcriptional control in bone cells. J Biol Chem. 1989 Jan 5;264(1):450–456. [PubMed] [Google Scholar]