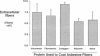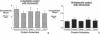Abstract
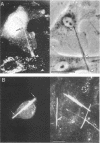
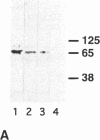
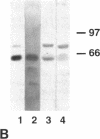
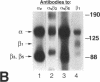
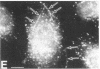
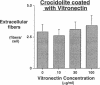
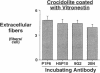
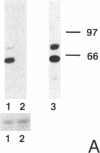
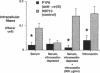
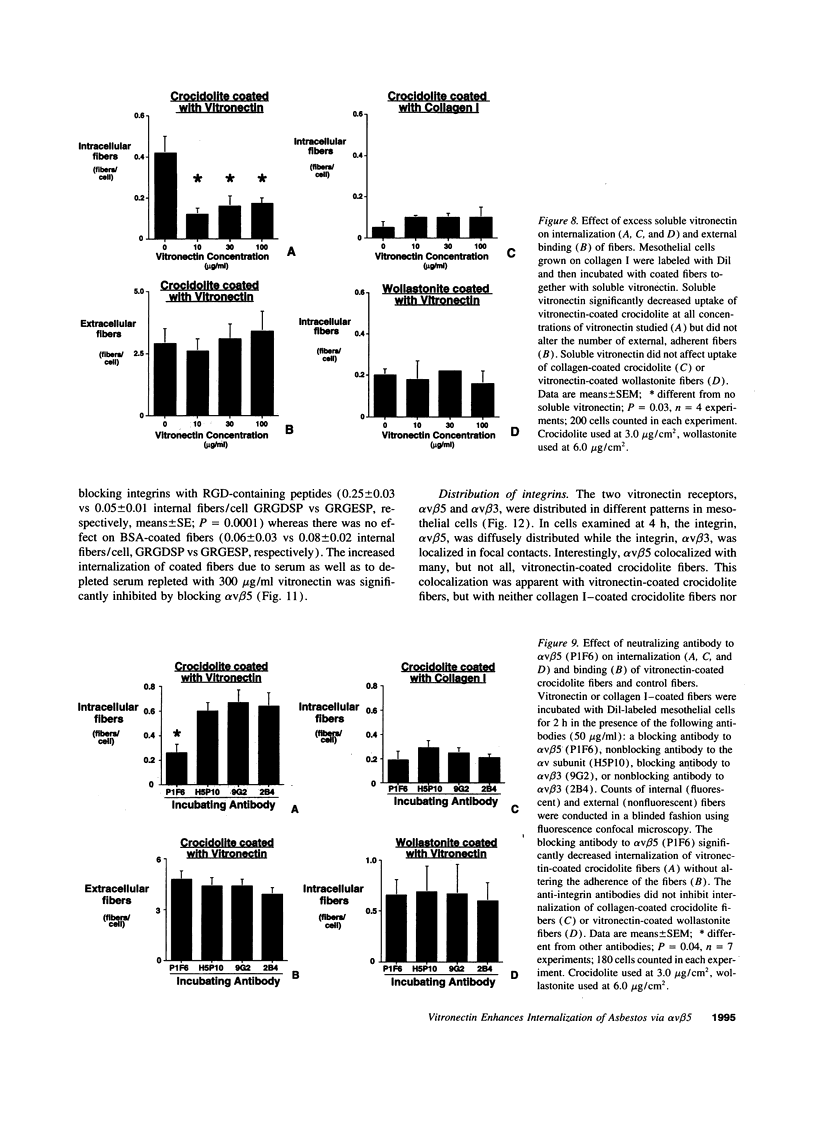
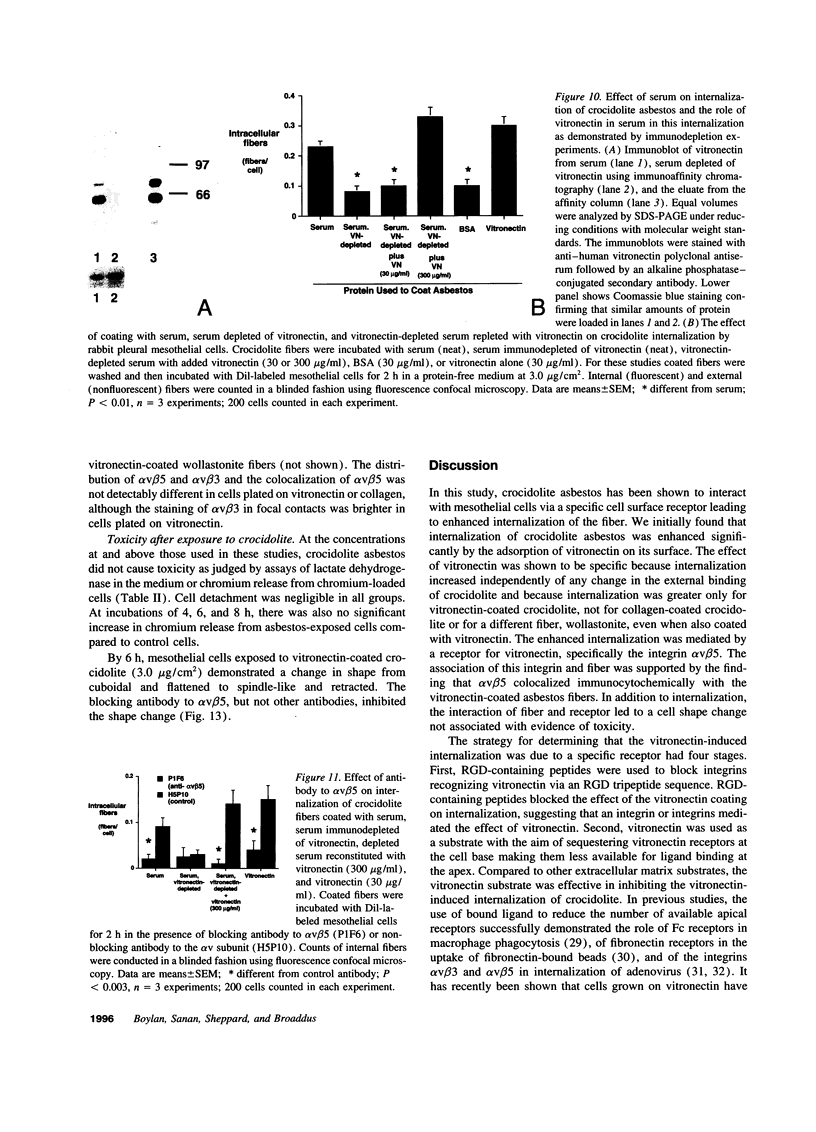
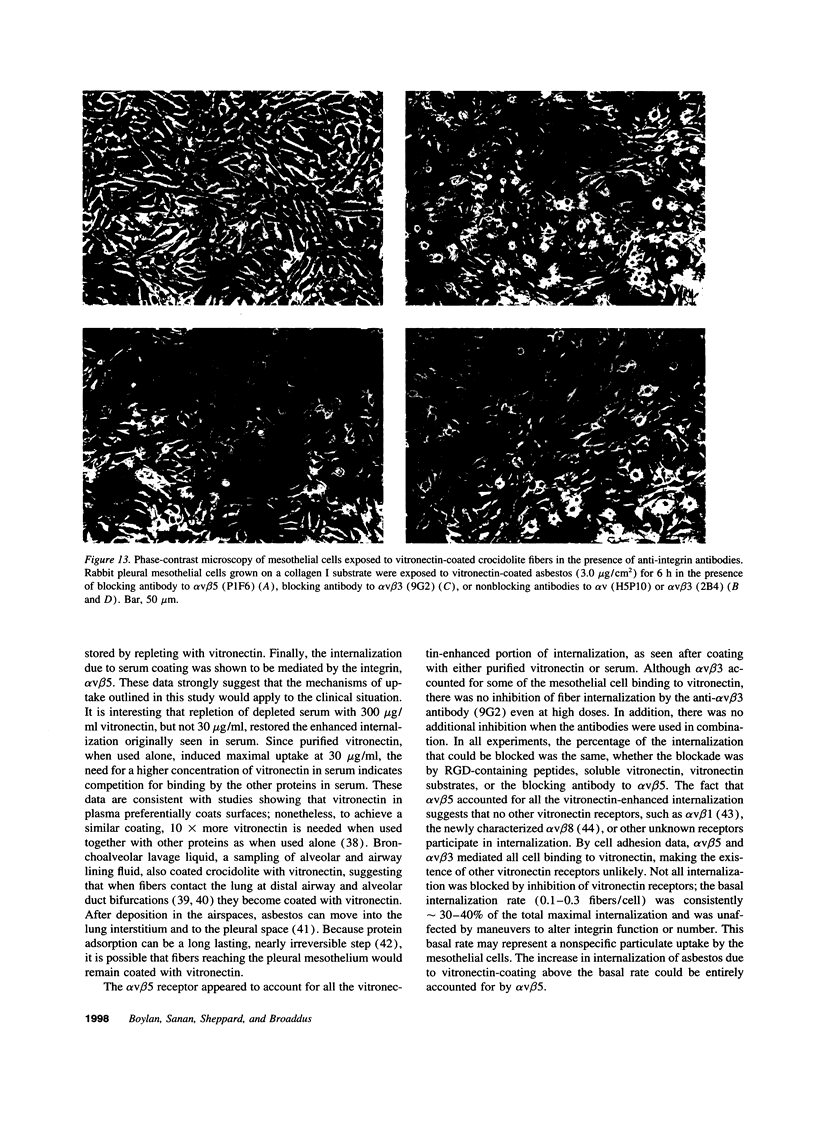
The mechanism by which pleural mesothelial cells, the likely progenitor cells of asbestos-induced mesothelioma, recognize and internalize crocidolite asbestos is unknown. Because incubation of asbestos fibers with serum increases their association with cells, we asked whether a protein coat on asbestos increased internalization of fibers via specific cellular receptors. Coating crocidolite with citronectin, but not with fibronectin or other proteins, increased fiber internalization by rabbit pleural mesothelial cells, as measured by a new technique using fluorescence confocal microscopy. Receptors for vitronectin, alpha v beta 3 and alpha v beta 5, were identified on mesothelial cells. Inhibiting vitronectin receptors by plating cells on a vitronectin substrate or incubating cells with excess soluble vitronectin reduced internalization of vitronectin-coated crocidolite. Inhibition of alpha v beta 5, but not alpha v beta 3, with blocking antibodies similarly reduced internalization. In addition, alpha v beta 5, but not alpha v beta 3, showed immunocytochemical colocalization with fibers. Of biologic relevance, coating crocidolite with serum also increased internalization via alpha v beta 5, an effect dependent on the vitronectin in serum. We conclude that pleural mesothelial cells recognize and internalize vitronectin- and serum-coated asbestos via the integrin alpha v beta 5. Since integrins initiate some of the same signaling pathways as does asbestos, our findings may provide insights into the mechanisms of asbestos-induced biologic effects.
Full text
PDF


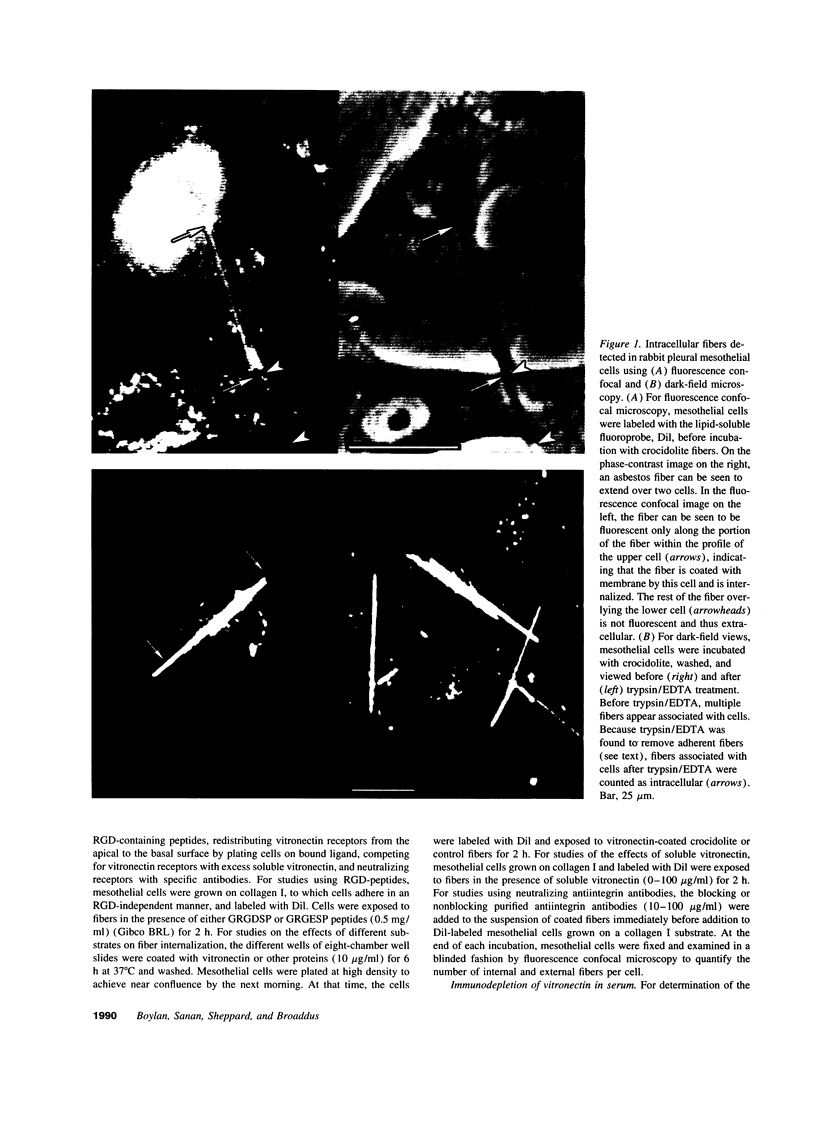

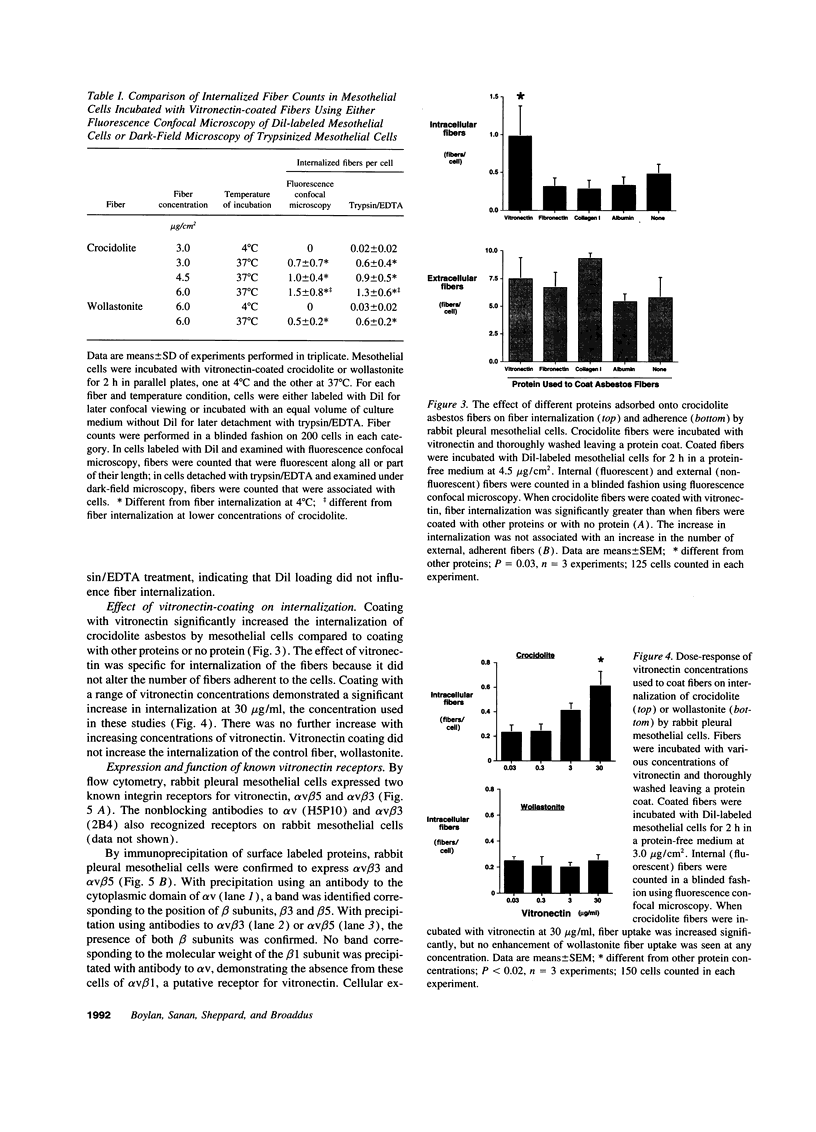
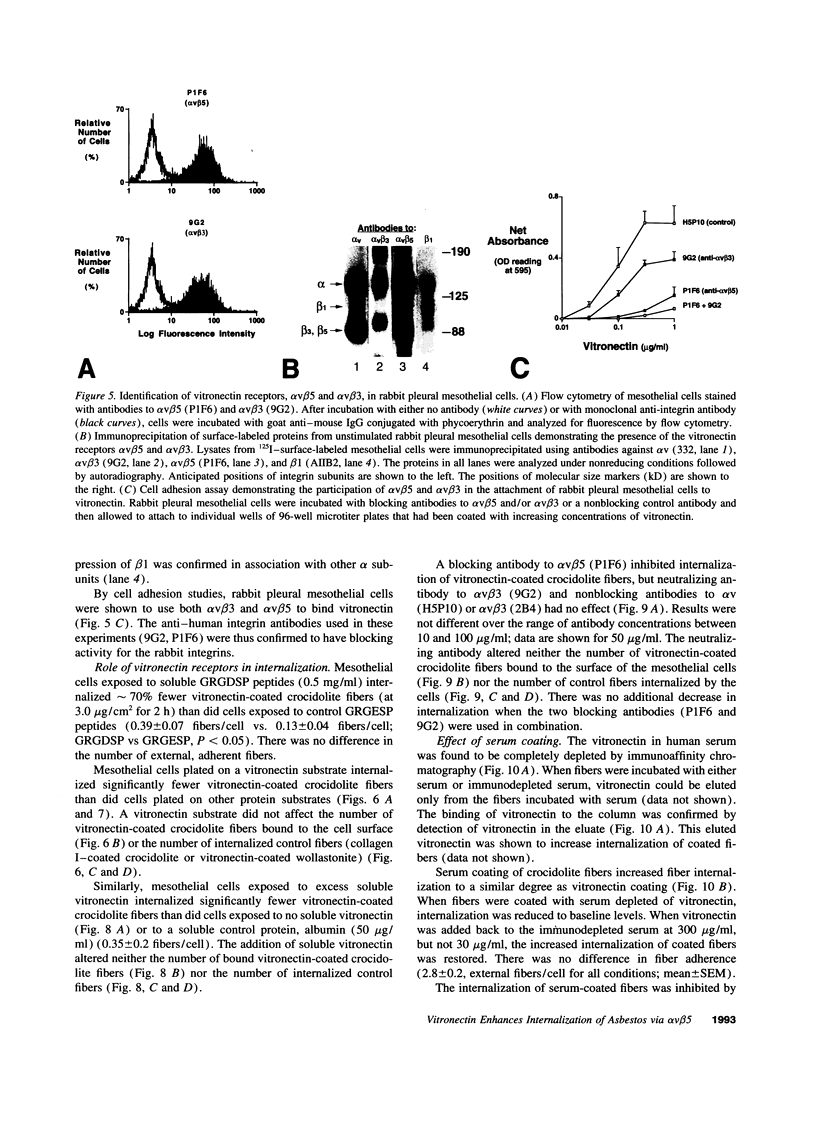
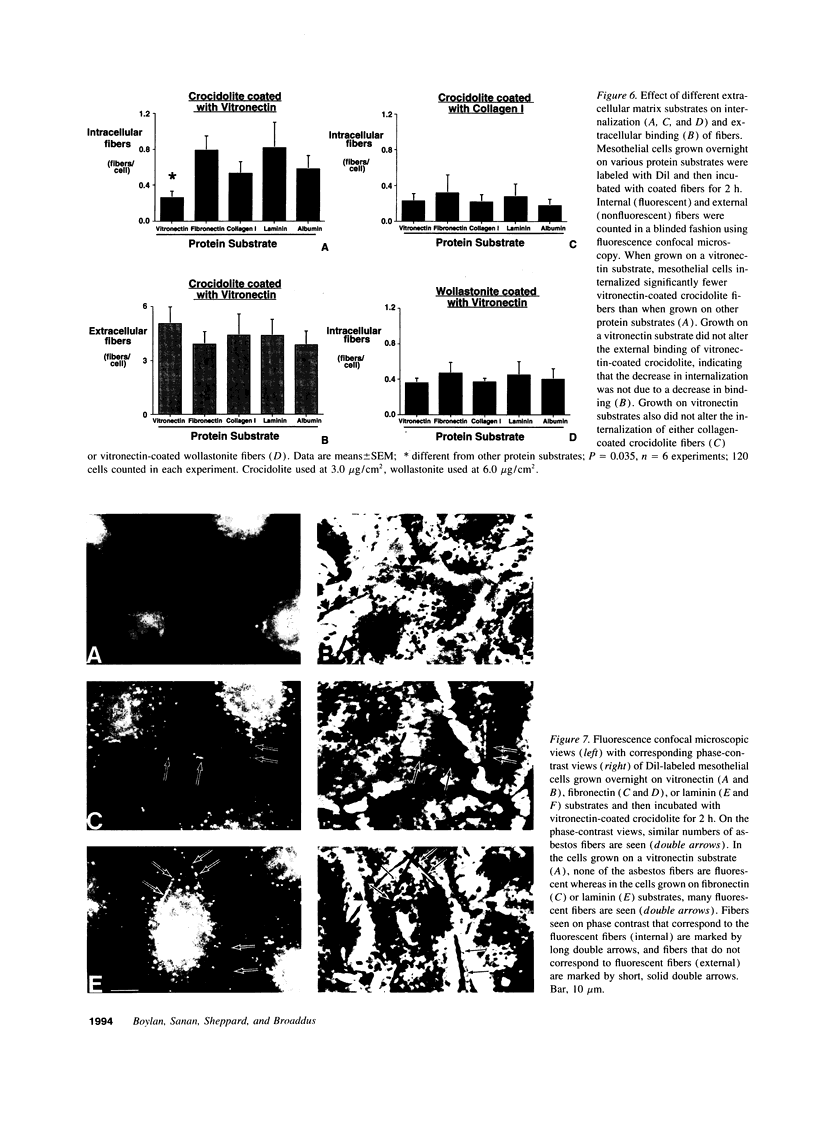




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel J. D., Fasy T. M., Kohtz D. S., Kohtz J. D., Johnson E. M. Asbestos fibers mediate transformation of monkey cells by exogenous plasmid DNA. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7670–7674. doi: 10.1073/pnas.85.20.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale M. D., Wohlfahrt L. A., Mosher D. F., Tomasini B., Sutton R. C. Identification of vitronectin as a major plasma protein adsorbed on polymer surfaces of different copolymer composition. Blood. 1989 Dec;74(8):2698–2706. [PubMed] [Google Scholar]
- Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990 Apr 15;265(11):5938–5941. [PubMed] [Google Scholar]
- Bossy B., Reichardt L. F. Chick integrin alpha V subunit molecular analysis reveals high conservation of structural domains and association with multiple beta subunits in embryo fibroblasts. Biochemistry. 1990 Nov 6;29(44):10191–10198. doi: 10.1021/bi00496a006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan A. M., Rüegg C., Kim K. J., Hébert C. A., Hoeffel J. M., Pytela R., Sheppard D., Goldstein I. M., Broaddus V. C. Evidence of a role for mesothelial cell-derived interleukin 8 in the pathogenesis of asbestos-induced pleurisy in rabbits. J Clin Invest. 1992 Apr;89(4):1257–1267. doi: 10.1172/JCI115710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Roe M. W. Deposition pattern of inorganic particles at the alveolar level in the lungs of rats and mice. Am Rev Respir Dis. 1983 Oct;128(4):724–729. doi: 10.1164/arrd.1983.128.4.724. [DOI] [PubMed] [Google Scholar]
- Chuntharapai A., Bodary S., Horton M., Kim K. J. Blocking monoclonal antibodies to alpha V beta 3 integrin: a unique epitope of alpha V beta 3 integrin is present on human osteoclasts. Exp Cell Res. 1993 Apr;205(2):345–352. doi: 10.1006/excr.1993.1096. [DOI] [PubMed] [Google Scholar]
- Cole R. W., Ault J. G., Hayden J. H., Rieder C. L. Crocidolite asbestos fibers undergo size-dependent microtubule-mediated transport after endocytosis in vertebrate lung epithelial cells. Cancer Res. 1991 Sep 15;51(18):4942–4947. [PubMed] [Google Scholar]
- Davis J. M. An electron microscope study of the response of mesothelial cells to the intrapleural injection of asbestos dust. Br J Exp Pathol. 1974 Feb;55(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Desai R., Richards R. J. The adsorption of biological macromolecules by mineral dusts. Environ Res. 1978 Jul;16(1-3):449–464. doi: 10.1016/0013-9351(78)90178-0. [DOI] [PubMed] [Google Scholar]
- Evans J. C., Evans R. J., Holmes A., Hounam R. F., Jones D. M., Morgan A., Walsh M. Studies on the deposition of inhaled fibrous material in the respiratory tract of the rat and its subsequent clearance using radioactive tracer techniques. 1. UICC crocidolite asbestos. Environ Res. 1973 Jun;6(2):180–201. doi: 10.1016/0013-9351(73)90032-7. [DOI] [PubMed] [Google Scholar]
- Fasske E. Pathogenesis of pulmonary fibrosis induced by chrysotile asbestos. Longitudinal light and electron microscopic studies on the rat model. Virchows Arch A Pathol Anat Histopathol. 1986;408(4):329–346. doi: 10.1007/BF00707692. [DOI] [PubMed] [Google Scholar]
- Friemann J., Müller K. M., Pott F. Mesothelial proliferation due to asbestos and man-made fibres. Experimental studies on rat omentum. Pathol Res Pract. 1990 Feb;186(1):117–123. doi: 10.1016/S0344-0338(11)81019-8. [DOI] [PubMed] [Google Scholar]
- Gan L., Savransky E. F., Fasy T. M., Johnson E. M. Transfection of human mesothelial cells mediated by different asbestos fiber types. Environ Res. 1993 Jul;62(1):28–42. doi: 10.1006/enrs.1993.1086. [DOI] [PubMed] [Google Scholar]
- Garcia J. G., Gray L. D., Dodson R. F., Callahan K. S. Asbestos-induced endothelial cell activation and injury. Demonstration of fiber phagocytosis and oxidant-dependent toxicity. Am Rev Respir Dis. 1988 Oct;138(4):958–964. doi: 10.1164/ajrccm/138.4.958. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. K., Mooney D. J., Vacanti J. P., Ingber D. E. Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Cell. 1994 Sep;5(9):967–975. doi: 10.1091/mbc.5.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen A., Schafer P. W., Lechner J. F., Stoner G. D., Trump B. F., Harris C. C. Cellular ingestion, toxic effects, and lesions observed in human bronchial epithelial tissue and cells cultured with asbestos and glass fibers. Int J Cancer. 1982 Sep 15;30(3):265–272. doi: 10.1002/ijc.2910300303. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ohgren Y., Ruoslahti E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4003–4007. doi: 10.1073/pnas.80.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. H., Janssen Y. M., Mossman B. T. Persistent induction of c-fos and c-jun expression by asbestos. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3299–3303. doi: 10.1073/pnas.90.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg T. W., Butterick C. J., Oshimura M., Brody A. R., Barrett J. C. Role of phagocytosis in Syrian hamster cell transformation and cytogenetic effects induced by asbestos and short and long glass fibers. Cancer Res. 1986 Nov;46(11):5795–5802. [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986 Jul;103(1):171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurand M. C., Bastie-Sigeac I., Bignon J., Stoebner P. Effect of chrysotile and crocidolite on the morphology and growth of rat pleural mesothelial cells. Environ Res. 1983 Apr;30(2):255–269. doi: 10.1016/0013-9351(83)90212-8. [DOI] [PubMed] [Google Scholar]
- Jaurand M. C., Kaplan H., Thiollet J., Pinchon M. C., Bernaudin J. F., Bignon J. Phagocytosis of chrysotile fibers by pleural mesothelial cells in culture. Am J Pathol. 1979 Mar;94(3):529–538. [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp D. W., Dunne M., Anderson J. A., Weitzman S. A., Dunn M. M. Serum promotes asbestos-induced injury to human pulmonary epithelial cells. J Lab Clin Med. 1990 Sep;116(3):289–297. [PubMed] [Google Scholar]
- Kamp D. W., Dunne M., Weitzman S. A., Dunn M. M. The interaction of asbestos and neutrophils injures cultured human pulmonary epithelial cells: role of hydrogen peroxide. J Lab Clin Med. 1989 Nov;114(5):604–612. [PubMed] [Google Scholar]
- Kuwahara M., Kuwahara M., Verma K., Ando T., Hemenway D. R., Kagan E. Asbestos exposure stimulates pleural mesothelial cells to secrete the fibroblast chemoattractant, fibronectin. Am J Respir Cell Mol Biol. 1994 Feb;10(2):167–176. doi: 10.1165/ajrcmb.10.2.8110473. [DOI] [PubMed] [Google Scholar]
- Lechner J. F., Tokiwa T., LaVeck M., Benedict W. F., Banks-Schlegel S., Yeager H., Jr, Banerjee A., Harris C. C. Asbestos-associated chromosomal changes in human mesothelial cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3884–3888. doi: 10.1073/pnas.82.11.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J. L., Kane A. B. Identification of asbestos fibers within single cells. Lab Invest. 1986 Aug;55(2):177–185. [PubMed] [Google Scholar]
- Malorni W., Iosi F., Falchi M., Donelli G. On the mechanism of cell internalization of chrysotile fibers: an immunocytochemical and ultrastructural study. Environ Res. 1990 Aug;52(2):164–177. doi: 10.1016/s0013-9351(05)80251-8. [DOI] [PubMed] [Google Scholar]
- Nishimura S. L., Sheppard D., Pytela R. Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J Biol Chem. 1994 Nov 18;269(46):28708–28715. [PubMed] [Google Scholar]
- Pailes W. H., Judy D. J., Resnick H., Castranova V. Relative effects of asbestos and wollastonite on alveolar macrophages. J Toxicol Environ Health. 1984;14(4):497–510. doi: 10.1080/15287398409530601. [DOI] [PubMed] [Google Scholar]
- Panetti T. S., McKeown-Longo P. J. The alpha v beta 5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem. 1993 Jun 5;268(16):11492–11495. [PubMed] [Google Scholar]
- Preissner K. T. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Manejias R. E., Nussenzweig V. Selective phagocytic paralysis induced by immobilized immune complexes. J Exp Med. 1975 Oct 1;142(4):827–838. doi: 10.1084/jem.142.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom W. N., Travis W. D., Brody A. R. Cellular and molecular basis of the asbestos-related diseases. Am Rev Respir Dis. 1991 Feb;143(2):408–422. doi: 10.1164/ajrccm/143.2.408. [DOI] [PubMed] [Google Scholar]
- Rüttner J. R., Lang A. B., Gut D. R., Wydler M. U. Morphological aspects of interactions between asbestos fibers and human mesothelial cell cytoskeleton. Exp Cell Biol. 1987;55(6):285–294. doi: 10.1159/000163430. [DOI] [PubMed] [Google Scholar]
- Scheule R. K., Holian A. Modification of asbestos bioactivity for the alveolar macrophage by selective protein adsorption. Am J Respir Cell Mol Biol. 1990 May;2(5):441–448. doi: 10.1165/ajrcmb/2.5.441. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Ingber D. E., Lawrence M., Springer T. A., Lechene C. Multiple integrins share the ability to induce elevation of intracellular pH. Exp Cell Res. 1991 Aug;195(2):533–535. doi: 10.1016/0014-4827(91)90407-l. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C., Ingber D. E. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Vestal D. J., Irwin S. V., Burke T. A., Cheresh D. A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990 Jul 5;265(19):11008–11013. [PubMed] [Google Scholar]
- Stanton M. F., Layard M., Tegeris A., Miller E., May M., Morgan E., Smith A. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981 Nov;67(5):965–975. [PubMed] [Google Scholar]
- Tomasini B. R., Mosher D. F. Vitronectin. Prog Hemost Thromb. 1991;10:269–305. [PubMed] [Google Scholar]
- Valerio F., Balducci D., Lazzarotto A. Adsorption of proteins by chrysotile and crocidolite: role of molecular weight and charge density. Environ Res. 1987 Dec;44(2):312–320. doi: 10.1016/s0013-9351(87)80240-2. [DOI] [PubMed] [Google Scholar]
- Valerio F., Balducci D., Scarabelli L. Selective adsorption of serum proteins by chrysotile and crocidolite. Environ Res. 1986 Dec;41(2):432–439. doi: 10.1016/s0013-9351(86)80137-2. [DOI] [PubMed] [Google Scholar]
- Viallat J. R., Raybuad F., Passarel M., Boutin C. Pleural migration of chrysotile fibers after intratracheal injection in rats. Arch Environ Health. 1986 Sep-Oct;41(5):282–286. doi: 10.1080/00039896.1986.9936697. [DOI] [PubMed] [Google Scholar]
- Wang N. S., Jaurand M. C., Magne L., Kheuang L., Pinchon M. C., Bignon J. The interactions between asbestos fibers and metaphase chromosomes of rat pleural mesothelial cells in culture. A scanning and transmission electron microscopic study. Am J Pathol. 1987 Feb;126(2):343–349. [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinacker A., Chen A., Agrez M., Cone R. I., Nishimura S., Wayner E., Pytela R., Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994 Mar 4;269(9):6940–6948. [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Filardo E. J., Cheresh D. A., Nemerow G. R. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994 Oct;127(1):257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Yatohgo T., Izumi M., Kashiwagi H., Hayashi M. Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct. 1988 Aug;13(4):281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]