Abstract
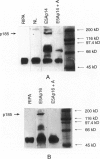
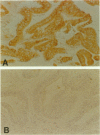
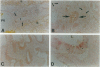
Immunohistochemical studies have suggested that the tyrosine kinase growth factor receptor p185neu is overexpressed in a high percentage of human cholangiocarcinomas. To establish the specificity and temporal relationship between the expression of this receptor in cholangiocarcinogenesis, we investigated c-neu expression in precancerous cholangiofibrotic tissue and subsequently derived primary and transplantable cholangiocarcinomas originated in the livers of furan-treated rats. Proliferated bile ductules formed in rat models of bile ductular hyperplasia and the cell types of normal adult rat liver were also analyzed for c-neu expression. c-neu expression was not detected in normal adult rat liver by either Western blotting, immunohistochemistry, or in situ hybridization. In comparison, all of the cholangiocarcinomas analyzed, which were characterized by intestinal-type mucin-producing neoplastic glands, exhibited a prominent band with a molecular weight 185 kd, corresponding to p185neu. Only the neoplastic glandular epithelia of the cholangiocarcinomas showed a strong immunoreactivity for p185neu, which was predominantly localized to their cell surface but also observed cytoplasmically. In situ hybridization further revealed the cytoplasm of the tumor glandular epithelial cells to be strongly positive for c-neu mRNA transcripts. Of particular interest was our finding that c-neu is expressed early in furan cholangiocarcinogenesis, being more pronounced in the metaplastic intestinal glands of cholangiofibrotic tissue than in hyperplastic biliary epithelial cells in either the same tissue or in hyperplastic bile ductule tissue. Our results demonstrate that c-neu overexpression is a prominent feature of intestinal-type cholangiocarcinomas as well as of metaplastic intestinal glands that precede their development and is detected at lower levels in hyperplastic biliary epithelia. The overexpression of c-neu in the metaplastic and malignant neoplastic glands also correlated with their increased proliferating cell nuclear antigen (PCNA) labeling indices relative to those of hyperplastic biliary ducts and ductules and also appeared to correlate with their intestinal glandular pattern of differentiation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg A., Tandon A. K., Sigurdsson H., Clark G. M., Fernö M., Fuqua S. A., Killander D., McGuire W. L. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990 Jul 15;50(14):4332–4337. [PubMed] [Google Scholar]
- Carver R. S., Sliwkowski M. X., Sitaric S., Russell W. E. Insulin regulates heregulin binding and ErbB3 expression in rat hepatocytes. J Biol Chem. 1996 Jun 7;271(23):13491–13496. doi: 10.1074/jbc.271.23.13491. [DOI] [PubMed] [Google Scholar]
- Chen Q., Seol D. W., Carr B., Zarnegar R. Co-expression and regulation of Met and Ron proto-oncogenes in human hepatocellular carcinoma tissues and cell lines. Hepatology. 1997 Jul;26(1):59–66. doi: 10.1002/hep.510260108. [DOI] [PubMed] [Google Scholar]
- Collier J. D., Guo K., Mathew J., May F. E., Bennett M. K., Corbett I. P., Bassendine M. F., Burt A. D. c-erbB-2 oncogene expression in hepatocellular carcinoma and cholangiocarcinoma. J Hepatol. 1992 Mar;14(2-3):377–380. doi: 10.1016/0168-8278(92)90186-s. [DOI] [PubMed] [Google Scholar]
- Elmore L. W., Sirica A. E. "Intestinal-type" of adenocarcinoma preferentially induced in right/caudate liver lobes of rats treated with furan. Cancer Res. 1993 Jan 15;53(2):254–259. [PubMed] [Google Scholar]
- Elmore L. W., Sirica A. E. Phenotypic characterization of metaplastic intestinal glands and ductular hepatocytes in cholangiofibrotic lesions rapidly induced in the caudate liver lobe of rats treated with furan. Cancer Res. 1991 Oct 15;51(20):5752–5759. [PubMed] [Google Scholar]
- Elmore L. W., Sirica A. E. Sequential appearance of intestinal mucosal cell types in the right and caudate liver lobes of furan-treated rats. Hepatology. 1992 Nov;16(5):1220–1226. [PubMed] [Google Scholar]
- Falck V. G., Gullick W. J. c-erbB-2 oncogene product staining in gastric adenocarcinoma. An immunohistochemical study. J Pathol. 1989 Oct;159(2):107–111. doi: 10.1002/path.1711590204. [DOI] [PubMed] [Google Scholar]
- Fernández J. L., Goyanes V., López-Fernández C., Buño I., Gosálvez J. Quantification of C-ERB-B2 gene amplification in breast cancer cells using fluorescence in situ hybridization and digital image analysis. Cancer Genet Cytogenet. 1996 Jan;86(1):18–21. doi: 10.1016/0165-4608(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Hughes C. M., Staddon S. L., Richman P. I., Gullick W. J., Lemoine N. R. The c-erb B-2 proto-oncogene in human pancreatic cancer. J Pathol. 1990 Jul;161(3):195–200. doi: 10.1002/path.1711610305. [DOI] [PubMed] [Google Scholar]
- Hilton D. A., West K. P. c-erbB-2 oncogene product expression and prognosis in gastric carcinoma. J Clin Pathol. 1992 May;45(5):454–456. doi: 10.1136/jcp.45.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ishii M., Vroman B., LaRusso N. F. Morphologic demonstration of receptor-mediated endocytosis of epidermal growth factor by isolated bile duct epithelial cells. Gastroenterology. 1990 May;98(5 Pt 1):1284–1291. doi: 10.1016/0016-5085(90)90346-3. [DOI] [PubMed] [Google Scholar]
- King C. R., Borrello I., Bellot F., Comoglio P., Schlessinger J. Egf binding to its receptor triggers a rapid tyrosine phosphorylation of the erbB-2 protein in the mammary tumor cell line SK-BR-3. EMBO J. 1988 Jun;7(6):1647–1651. doi: 10.1002/j.1460-2075.1988.tb02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka S., Kurashina M., Tsubone M., Hachisuka K., Yasui A. Significance of intestinal metaplasia for the evolution of cancer in the biliary tract. Cancer. 1984 Nov 15;54(10):2277–2285. doi: 10.1002/1097-0142(19841115)54:10<2277::aid-cncr2820541037>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Maronpot R. R., Giles H. D., Dykes D. J., Irwin R. D. Furan-induced hepatic cholangiocarcinomas in Fischer 344 rats. Toxicol Pathol. 1991;19(4 Pt 2):561–570. doi: 10.1177/019262339101900401. [DOI] [PubMed] [Google Scholar]
- Mathis G. A., Sirica A. E. Effects of medium and substratum conditions on the rates of DNA synthesis in primary cultures of bile ductular epithelial cells. In Vitro Cell Dev Biol. 1990 Feb;26(2):113–118. doi: 10.1007/BF02624101. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Fujii H., Michalopoulos G., Fung J. J., Demetris A. J. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology. 1994 Aug;20(2):376–382. [PubMed] [Google Scholar]
- Mortelmans K., Haworth S., Lawlor T., Speck W., Tainer B., Zeiger E. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 1986;8 (Suppl 7):1–119. [PubMed] [Google Scholar]
- Motojima K., Komuta K., Hiasa A., Tsuribune T., Hashimoto T., Tsunoda T., Kanematsu T. [Evaluation of immunoreactivity to erbB-2 protein as a marker of prognosis in bile duct carcinoma]. Nihon Geka Gakkai Zasshi. 1992 Sep;93(9):952–955. [PubMed] [Google Scholar]
- Nonomura A., Ohta G., Nakanuma Y., Izumi R., Mizukami Y., Matsubara F., Hayashi M., Watanabe K., Takayanagi N. Simultaneous detection of epidermal growth factor receptor (EGF-R), epidermal growth factor (EGF) and ras p21 in cholangiocarcinoma by an immunocytochemical method. Liver. 1988 Jun;8(3):157–166. doi: 10.1111/j.1600-0676.1988.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Polimeno L., Azzarone A., Zeng Q. H., Panella C., Subbotin V., Carr B., Bouzahzah B., Francavilla A., Starzl T. E. Cell proliferation and oncogene expression after bile duct ligation in the rat: evidence of a specific growth effect on bile duct cells. Hepatology. 1995 Apr;21(4):1070–1078. [PMC free article] [PubMed] [Google Scholar]
- Presnell S. C., Stolz D. B., Mars W. M., Jo M., Michalopoulos G. K., Strom S. C. Modifications of the hepatocyte growth factor/c-met pathway by constitutive expression of transforming growth factor-alpha in rat liver epithelial cells. Mol Carcinog. 1997 Apr;18(4):244–255. [PubMed] [Google Scholar]
- Riese D. J., Kim E. D., Elenius K., Buckley S., Klagsbrun M., Plowman G. D., Stern D. F. The epidermal growth factor receptor couples transforming growth factor-alpha, heparin-binding epidermal growth factor-like factor, and amphiregulin to Neu, ErbB-3, and ErbB-4. J Biol Chem. 1996 Aug 16;271(33):20047–20052. doi: 10.1074/jbc.271.33.20047. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Demetris A. J., Gollin S. M., Storto P. D., Bedford H. M., Altarac S., Iwatsuki S., Herberman R. B., Whiteside T. L. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992 Sep 9;52(2):252–260. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- Sirica A. E. Biliary proliferation and adaptation in furan-induced rat liver injury and carcinogenesis. Toxicol Pathol. 1996 Jan-Feb;24(1):90–99. doi: 10.1177/019262339602400113. [DOI] [PubMed] [Google Scholar]
- Sirica A. E. Biology of biliary epithelial cells. Prog Liver Dis. 1992;10:63–87. [PubMed] [Google Scholar]
- Sirica A. E., Cole S. L., Williams T. A unique rat model of bile ductular hyperplasia in which liver is almost totally replaced with well-differentiated bile ductules. Am J Pathol. 1994 Jun;144(6):1257–1268. [PMC free article] [PubMed] [Google Scholar]
- Sirica A. E., Gainey T. W. A new rat bile ductular epithelial cell culture model characterized by the appearance of polarized bile ducts in vitro. Hepatology. 1997 Sep;26(3):537–549. doi: 10.1002/hep.510260302. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voravud N., Foster C. S., Gilbertson J. A., Sikora K., Waxman J. Oncogene expression in cholangiocarcinoma and in normal hepatic development. Hum Pathol. 1989 Dec;20(12):1163–1168. doi: 10.1016/s0046-8177(89)80006-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Goldsworthy T. L., Popp J. A., Butterworth B. E. Evaluation of genotoxicity, pathological lesions, and cell proliferation in livers of rats and mice treated with furan. Environ Mol Mutagen. 1992;19(3):209–222. doi: 10.1002/em.2850190305. [DOI] [PubMed] [Google Scholar]
- Worthylake R., Wiley H. S. Structural aspects of the epidermal growth factor receptor required for transmodulation of erbB-2/neu. J Biol Chem. 1997 Mar 28;272(13):8594–8601. doi: 10.1074/jbc.272.13.8594. [DOI] [PubMed] [Google Scholar]
- Wu M. S., Shun C. T., Wang H. P., Sheu J. C., Lee W. J., Wang T. H., Lin J. T. Genetic alterations in gastric cancer: relation to histological subtypes, tumor stage, and Helicobacter pylori infection. Gastroenterology. 1997 May;112(5):1457–1465. doi: 10.1016/s0016-5085(97)70071-4. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y., Friess H., Kobrin M. S., Büchler M., Kunz J., Beger H. G., Korc M. Overexpression of HER2/neu oncogene in human pancreatic carcinoma. Hum Pathol. 1993 Oct;24(10):1127–1134. doi: 10.1016/0046-8177(93)90194-l. [DOI] [PubMed] [Google Scholar]