Abstract
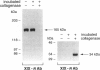
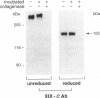
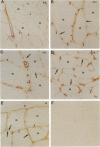
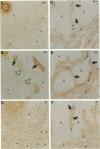
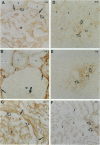
Nineteen types, the product of 33 genes, comprise the collagen family of proteins. Types I, II, III, V, and XI constitute the fibrillar collagens, whereas types IV, VI to X, and XII to XIX represent the structurally diverse, nonfibrillar members. Type XIX collagen was discovered from the sequence of rhabdomyosarcoma cDNA clones. The type XIX chain consists of 1142 amino acids that contribute primarily to a unique five subdomain triple-helical region. To characterize the protein, to determine the tissue distribution, and to provide some insight into its function, we generated two type XIX-specific polyclonal antibodies. One was directed against a recombinant molecule containing amino-terminal sequences, and the second was derived from a synthetic peptide corresponding to most of the short carboxy terminus. These antibodies were used in immunoblot assays of rhabdomyosarcoma cell/matrix homogenates to identify a 165-kd disulfide-bonded and bacterial collagenase-sensitive protein. Immunohistochemical analysis of type XIX collagen was performed for human skeletal muscle, spleen, prostate, kidney, liver, placenta, colon, and skin. In contrast to Northern blot hybridizations, which showed very low levels of the 12-kb transcript in few tissues, the protein was found in all tissues examined. The type XIX collagen distribution was restricted to vascular, neuronal, mesenchymal, and some epithelial basement membrane zones, which is similar to the profile recently established (Ref. 8) and further extended here for type XV collagen. Nevertheless, localization of type XIX exhibited significant differences from type XV collagen that were particularly evident in the kidney, liver, and spleen. This report, in conjunction with the type XV results and other studies of type XVIII collagen, indicates the existence of a new collagen subgroup founded on their widespread presence in basement membrane zones regardless of chain homology. In addition to their role in basement membrane-stromal interactions, the pronounced vascular association suggests involvement of these related collagen types with angiogenic and pathological processes.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apaja-Sarkkinen M., Alavaikko M., Karttunen T., Autio-Harmainen H. Basement membrane proteins in the spleen: immunohistochemical demonstration and relation to reticulin. Histopathology. 1986 Mar;10(3):295–302. doi: 10.1111/j.1365-2559.1986.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Baloch Z., Klapper J., Buchanan L., Schwartz M., Amenta P. S. Ontogenesis of the murine hepatic extracellular matrix: an immunohistochemical study. Differentiation. 1992 Nov;51(3):209–218. doi: 10.1111/j.1432-0436.1992.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H., Stein U., Remberger K. Differential expression of alpha 6 and alpha 2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol. 1993 Mar;24(3):243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Timpl R. The collagen superfamily. Int Arch Allergy Immunol. 1995 Aug;107(4):484–490. doi: 10.1159/000237090. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Shyu A. B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995 Nov;20(11):465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Gordon M. K., Olsen B. R. The contribution of collagenous proteins to tissue-specific matrix assemblies. Curr Opin Cell Biol. 1990 Oct;2(5):833–838. doi: 10.1016/0955-0674(90)90080-x. [DOI] [PubMed] [Google Scholar]
- Grässel S., Timpl R., Tan E. M., Chu M. L. Biosynthesis and processing of type XVI collagen in human fibroblasts and smooth muscle cells. Eur J Biochem. 1996 Dec 15;242(3):576–584. doi: 10.1111/j.1432-1033.1996.0576r.x. [DOI] [PubMed] [Google Scholar]
- Inoguchi K., Yoshioka H., Khaleduzzaman M., Ninomiya Y. The mRNA for alpha 1(XIX) collagen chain, a new member of FACITs, contains a long unusual 3' untranslated region and displays many unique splicing variants. J Biochem. 1995 Jan;117(1):137–146. doi: 10.1093/oxfordjournals.jbchem.a124700. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Peltz S. W. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- Konomi H., Hayashi T., Nakayasu K., Arima M. Localization of type V collagen and type IV collagen in human cornea, lung, and skin. Immunohistochemical evidence by anti-collagen antibodies characterized by immunoelectroblotting. Am J Pathol. 1984 Sep;116(3):417–426. [PMC free article] [PubMed] [Google Scholar]
- Liakka A., Apaja-Sarkkinen M., Karttunen T., Autio-Harmainen H. Distribution of laminin and types IV and III collagen in fetal, infant and adult human spleens. Cell Tissue Res. 1991 Feb;263(2):245–252. doi: 10.1007/BF00318766. [DOI] [PubMed] [Google Scholar]
- Ljubimov A. V., Bartek J., Couchman J. R., Kapuller L. L., Veselov V. V., Kovarik J., Perevoshchikov A. G., Krutovskikh V. A. Distribution of individual components of basement membrane in human colon polyps and adenocarcinomas as revealed by monoclonal antibodies. Int J Cancer. 1992 Feb 20;50(4):562–566. doi: 10.1002/ijc.2910500412. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- Muragaki Y., Timmons S., Griffith C. M., Oh S. P., Fadel B., Quertermous T., Olsen B. R. Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8763–8767. doi: 10.1073/pnas.92.19.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dion A. S., Abraham V., Amenta P. S. Type XV collagen exhibits a widespread distribution in human tissues but a distinct localization in basement membrane zones. Cell Tissue Res. 1996 Dec;286(3):493–505. doi: 10.1007/s004410050719. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Howard P. S., Jelen A. M., Dion A. S., Macarak E. J. Duplication of type IV collagen COOH-terminal repeats and species-specific expression of alpha 1(IV) and alpha 2(IV) collagen genes. J Biol Chem. 1987 Jul 5;262(19):9231–9238. [PubMed] [Google Scholar]
- Myers J. C., Sun M. J., D'Ippolito J. A., Jabs E. W., Neilson E. G., Dion A. S. Human cDNA clones transcribed from an unusually high-molecular-weight RNA encode a new collagen chain. Gene. 1993 Jan 30;123(2):211–217. doi: 10.1016/0378-1119(93)90126-n. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Yang H., D'Ippolito J. A., Presente A., Miller M. K., Dion A. S. The triple-helical region of human type XIX collagen consists of multiple collagenous subdomains and exhibits limited sequence homology to alpha 1(XVI). J Biol Chem. 1994 Jul 15;269(28):18549–18557. [PubMed] [Google Scholar]
- O'Reilly M. S., Boehm T., Shing Y., Fukai N., Vasios G., Lane W. S., Flynn E., Birkhead J. R., Olsen B. R., Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997 Jan 24;88(2):277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Pan T. C., Zhang R. Z., Mattei M. G., Timpl R., Chu M. L. Cloning and chromosomal location of human alpha 1(XVI) collagen. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6565–6569. doi: 10.1073/pnas.89.14.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Engvall E., Butkowski R., Hunter D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990 Oct;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H., Zhang H., Ramirez F., Mattei M. G., Moradi-Ameli M., van der Rest M., Gordon M. K. Synteny between the loci for a novel FACIT-like collagen locus (D6S228E) and alpha 1 (IX) collagen (COL9A1) on 6q12-q14 in humans. Genomics. 1992 Jul;13(3):884–886. doi: 10.1016/0888-7543(92)90176-s. [DOI] [PubMed] [Google Scholar]