Abstract
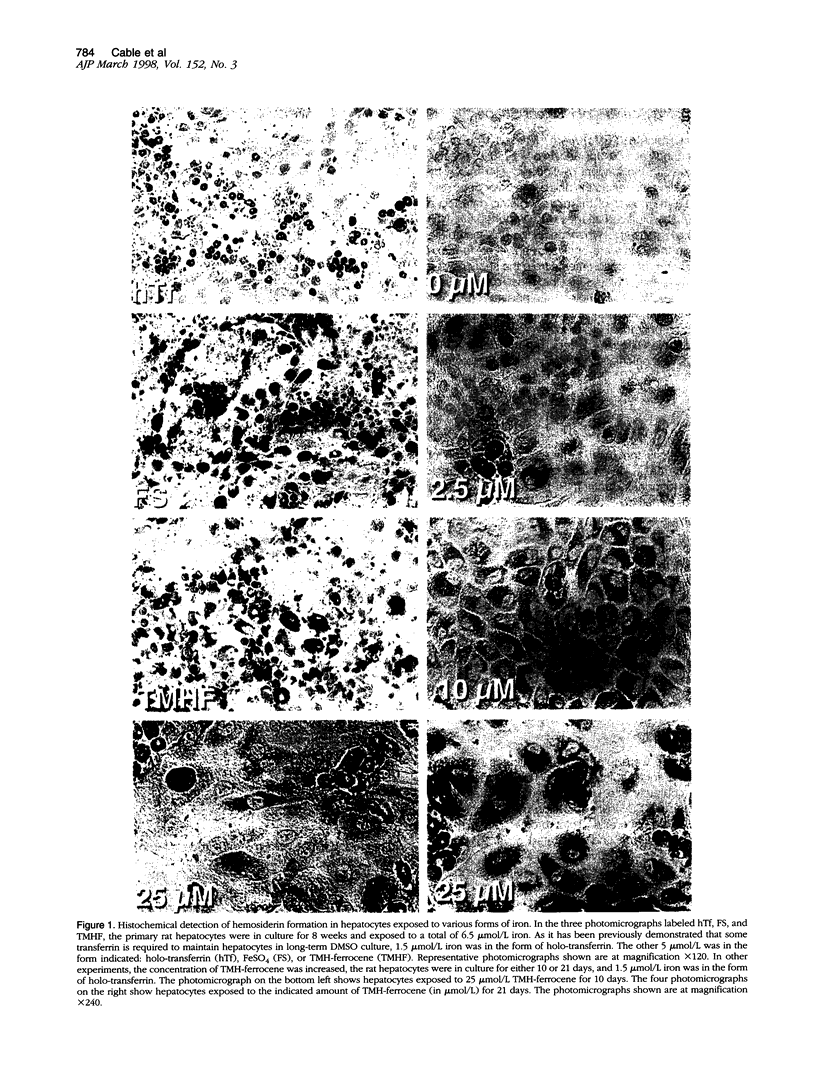
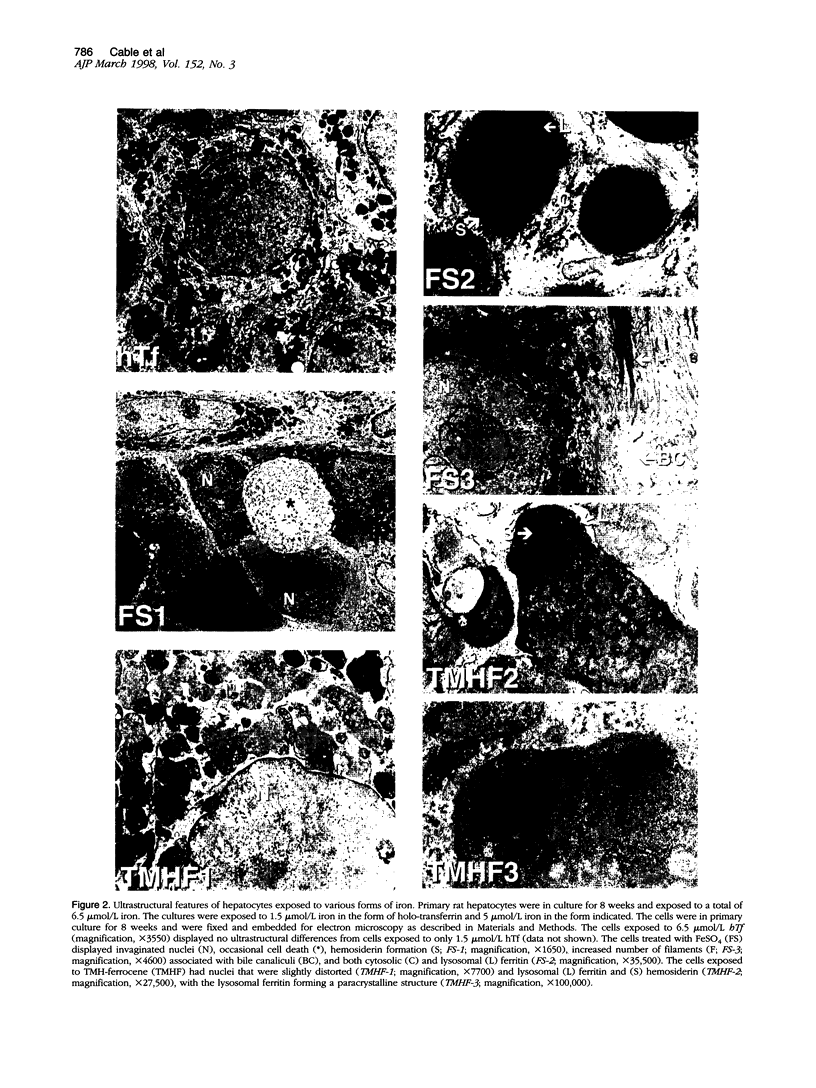
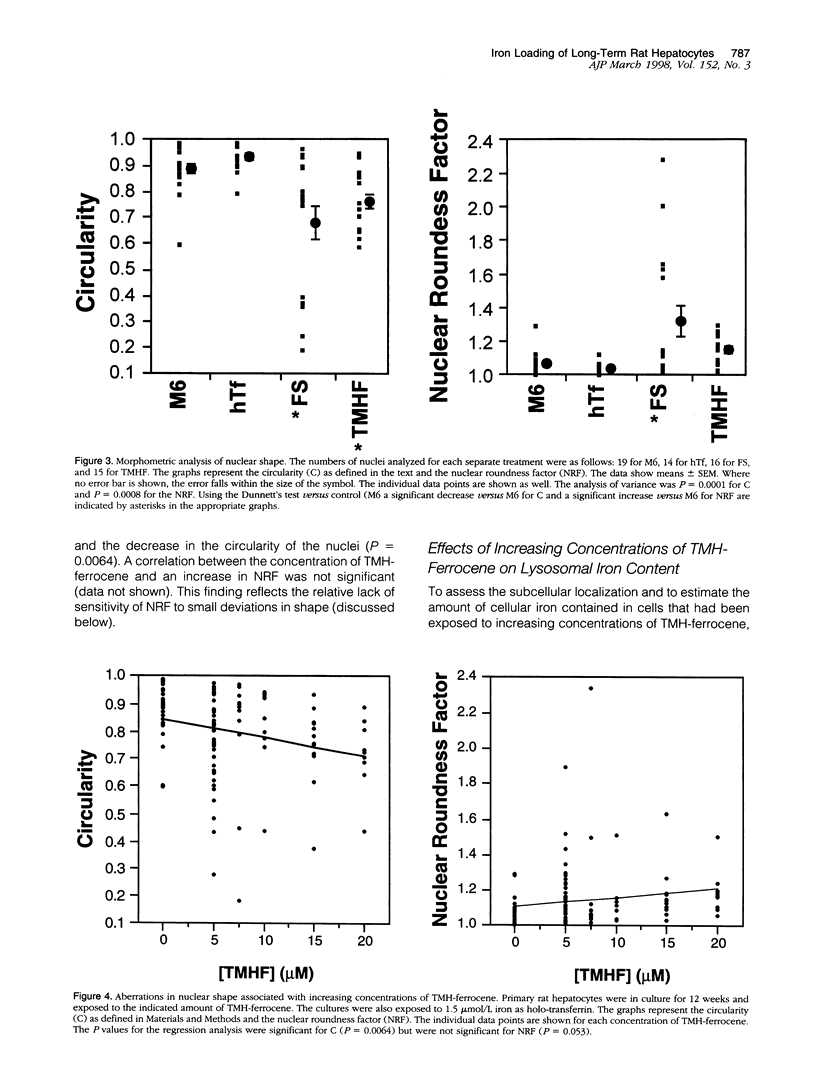
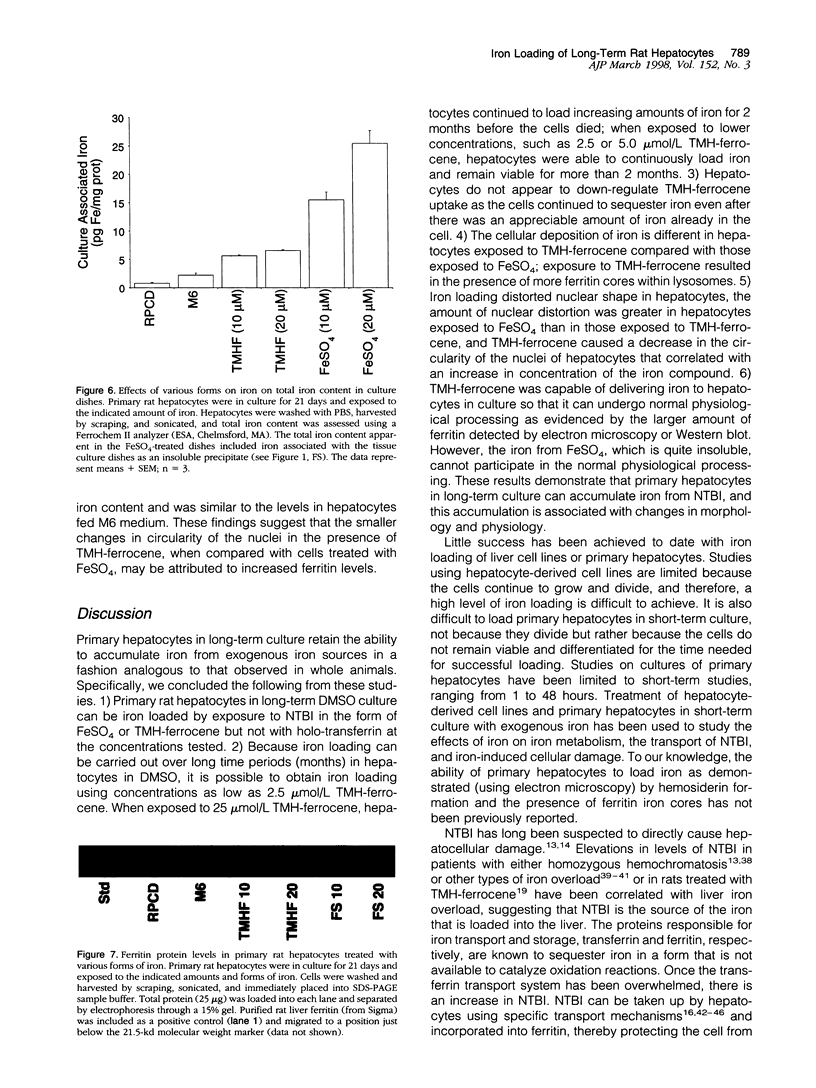
We have previously shown that hepatocytes in long-term dimethylsulfoxide (DMSO) culture, fed a chemically defined medium, are highly differentiated and an excellent in vitro model of adult liver. Hepatocytes in long-term DMSO culture can be iron loaded by exposure to non-transferrin-bound iron (NTBI) in the form of ferrous sulfate (FeSO4), ferric nitrilotriacetate, or trimethylhexanoyl (TMH)-ferrocene. Holotransferrin, at equivalent times and concentrations, was unable to load hepatocytes. Of the iron compounds tested, TMH-ferrocene most accurately simulated the morphological features of iron-loaded hepatocytes in vivo. When exposed to 25 micromol/L TMH-ferrocene, hepatocytes loaded increasing amounts of iron for 2 months before the cells died. When exposed to lower concentrations of TMH-ferrocene (as low as 2.5 micromol/L), hepatocytes continuously loaded iron and remained viable for more than 2 months. The cellular deposition of iron was different in hepatocytes exposed to TMH-ferrocene compared with those exposed to FeSO4; exposure to TMH-ferrocene resulted in the presence of more ferritin cores within lysosomes than were seen with FeSO4. When the concentration of TMH-ferrocene was increased, a greater number of ferritin cores were observed within the lysosome, and total cellular ferritin, as assessed by Western blot, increased. The formation of hemosiderin was also observed. Furthermore, nuclear shape was distorted in iron-loaded hepatocytes. The extent of deviation from circularity in the nucleus correlated with increasing concentrations of TMH-ferrocene and was greater in hepatocytes exposed to FeSO4 than an equivalent concentration of TMH-ferrocene. The deviation from circularity was smallest in hepatocytes that contained well formed ferritin cores and increased in hepatocytes that contained greater amounts of hemosiderin. Furthermore, in hepatocytes treated with FeSO4, a large amount of cell-associated iron was detected but without a significant increase in the total amount of ferritin. The deviation from circularity was the largest in FeSO4-treated hepatocytes, indicating that iron not properly incorporated into ferritin caused more cellular damage. We conclude that iron-loaded hepatocytes in long-term DMSO culture represent a flexible system for studying the effects of chronic iron loading on hepatocytes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo A., Kosaryan M., MacDowell A., Wickens D., Puri S., Wonke B., Hoffbrand A. V. A novel delivery system for continuous desferrioxamine infusion in transfusional iron overload. Br J Haematol. 1996 Jun;93(4):835–837. doi: 10.1046/j.1365-2141.1996.d01-1743.x. [DOI] [PubMed] [Google Scholar]
- Bacon B. R., Healey J. F., Brittenham G. M., Park C. H., Nunnari J., Tavill A. S., Bonkovsky H. L. Hepatic microsomal function in rats with chronic dietary iron overload. Gastroenterology. 1986 Jun;90(6):1844–1853. doi: 10.1016/0016-5085(86)90251-9. [DOI] [PubMed] [Google Scholar]
- Barisani D., Berg C. L., Wessling-Resnick M., Gollan J. L. Evidence for a low Km transporter for non-transferrin-bound iron in isolated rat hepatocytes. Am J Physiol. 1995 Oct;269(4 Pt 1):G570–G576. doi: 10.1152/ajpgi.1995.269.4.G570. [DOI] [PubMed] [Google Scholar]
- Batey R. G., Lai Chung Fong P., Shamir S., Sherlock S. A non-transferrin-bound serum iron in idiopathic hemochromatosis. Dig Dis Sci. 1980 May;25(5):340–346. doi: 10.1007/BF01308057. [DOI] [PubMed] [Google Scholar]
- Batey R. G., Shamir S., Wilms J. Properties and hepatic metabolism of non-transferrin-bound iron. Dig Dis Sci. 1981 Dec;26(12):1084–1088. doi: 10.1007/BF01295972. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour E. S., Ward L. K., Cornman G. A., Isom H. C. Tumor necrosis factor-alpha-induced apoptosis in hepatocytes in long-term culture. Am J Pathol. 1996 Feb;148(2):485–495. [PMC free article] [PubMed] [Google Scholar]
- Brissot P., Wright T. L., Ma W. L., Weisiger R. A. Efficient clearance of non-transferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J Clin Invest. 1985 Oct;76(4):1463–1470. doi: 10.1172/JCI112125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton R. S., Bacon B. R., Recknagel R. O. Lipid peroxidation and associated hepatic organelle dysfunction in iron overload. Chem Phys Lipids. 1987 Nov-Dec;45(2-4):207–239. doi: 10.1016/0009-3084(87)90066-1. [DOI] [PubMed] [Google Scholar]
- Britton R. S. Metal-induced hepatotoxicity. Semin Liver Dis. 1996 Feb;16(1):3–12. doi: 10.1055/s-2007-1007214. [DOI] [PubMed] [Google Scholar]
- Britton R. S., O'Neill R., Bacon B. R. Hepatic mitochondrial malondialdehyde metabolism in rats with chronic iron overload. Hepatology. 1990 Jan;11(1):93–97. doi: 10.1002/hep.1840110116. [DOI] [PubMed] [Google Scholar]
- Cable E. E., Isom H. C. Exposure of primary rat hepatocytes in long-term DMSO culture to selected transition metals induces hepatocyte proliferation and formation of duct-like structures. Hepatology. 1997 Dec;26(6):1444–1457. doi: 10.1002/hep.510260611. [DOI] [PubMed] [Google Scholar]
- Cammisa H. M., Isom H. C., Greene F. E. Hormonal regulation of pseudocholinesterase activity in cultured rat hepatocytes. Endocrinology. 1988 Mar;122(3):991–996. doi: 10.1210/endo-122-3-991. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. D. Adaptation in iron metabolism. Am J Clin Nutr. 1990 Feb;51(2):301–308. doi: 10.1093/ajcn/51.2.301. [DOI] [PubMed] [Google Scholar]
- Diamond D. A., Berry S. J., Umbricht C., Jewett H. J., Coffey D. S. Computerized image analysis of nuclear shape as a prognostic factor for prostatic cancer. Prostate. 1982;3(4):321–332. doi: 10.1002/pros.2990030402. [DOI] [PubMed] [Google Scholar]
- Düllmann J., Wulfhekel U., Nielsen P., Heinrich H. C. Iron overload of the liver by trimethylhexanoylferrocene in rats. Acta Anat (Basel) 1992;143(2):96–108. doi: 10.1159/000147235. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M., Bell J. D., Halliwell B., Aruoma O. I., Bomford A., Sadler P. J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Mar 15;264(8):4417–4422. [PubMed] [Google Scholar]
- Harford J. B., Klausner R. D. Coordinate post-transcriptional regulation of ferritin and transferrin receptor expression: the role of regulated RNA-protein interaction. Enzyme. 1990;44(1-4):28–41. doi: 10.1159/000468745. [DOI] [PubMed] [Google Scholar]
- Hu J. M., Camper S. A., Tilghman S. M., Miller T., Georgoff I., Serra R., Isom H. C. Functional analyses of albumin expression in a series of hepatocyte cell lines and in primary hepatocytes. Cell Growth Differ. 1992 Sep;3(9):577–588. [PubMed] [Google Scholar]
- Ingber D. E., Dike L., Hansen L., Karp S., Liley H., Maniotis A., McNamee H., Mooney D., Plopper G., Sims J. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int Rev Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- Isom H. C. DNA synthesis in isolated hepatocytes infected with herpesviruses. Virology. 1980 May;103(1):199–216. doi: 10.1016/0042-6822(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Isom H. C., Secott T., Georgoff I., Woodworth C., Mummaw J. Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci U S A. 1985 May;82(10):3252–3256. doi: 10.1073/pnas.82.10.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom I., Georgoff I., Salditt-Georgieff M., Darnell J. E., Jr Persistence of liver-specific messenger RNA in cultured hepatocytes: different regulatory events for different genes. J Cell Biol. 1987 Dec;105(6 Pt 2):2877–2885. doi: 10.1083/jcb.105.6.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Karim O. M., Seki N., Pienta K. J., Mostwin J. L. The effect of age on the response of the detrusor to intracellular mechanical stimulus: DNA replication and the cell actin matrix. J Cell Biochem. 1992 Apr;48(4):373–384. doi: 10.1002/jcb.240480406. [DOI] [PubMed] [Google Scholar]
- Kennard M. L., Richardson D. R., Gabathuler R., Ponka P., Jefferies W. A. A novel iron uptake mechanism mediated by GPI-anchored human p97. EMBO J. 1995 Sep 1;14(17):4178–4186. doi: 10.1002/j.1460-2075.1995.tb00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lash A., Saleem A. Iron metabolism and its regulation. A review. Ann Clin Lab Sci. 1995 Jan-Feb;25(1):20–30. [PubMed] [Google Scholar]
- Longueville A., Crichton R. R. An animal model of iron overload and its application to study hepatic ferritin iron mobilization by chelators. Biochem Pharmacol. 1986 Nov 1;35(21):3669–3678. doi: 10.1016/0006-2952(86)90650-7. [DOI] [PubMed] [Google Scholar]
- Lynch S. R., Skikne B. S., Cook J. D. Food iron absorption in idiopathic hemochromatosis. Blood. 1989 Nov 1;74(6):2187–2193. [PubMed] [Google Scholar]
- Martínez-Torres C., Leets I., Taylor P., Ramírez J., del Valle Camacho M., Layrisse M. Heme, ferritin and vegetable iron absorption in humans from meals denatured of heme iron during the cooking of beef. J Nutr. 1986 Sep;116(9):1720–1725. doi: 10.1093/jn/116.9.1720. [DOI] [PubMed] [Google Scholar]
- Mohler J. L., Partin A. W., Lohr W. D., Coffey D. S. Nuclear roundness factor measurement for assessment of prognosis of patients with prostatic carcinoma. I. Testing of a digitization system. J Urol. 1988 May;139(5):1080–1084. doi: 10.1016/s0022-5347(17)42791-1. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard U., Liem H. H., Grasso J. A., Giffhorn-Katz S., DeFalco M. G., Katz N. R. Increase in surface expression of transferrin receptors on cultured hepatocytes of adult rats in response to iron deficiency. J Biol Chem. 1988 Oct 15;263(29):14753–14756. [PubMed] [Google Scholar]
- Nielsen P., Düllmann J., Wulfhekel U., Heinrich H. C. Non-transferrin-bound-iron in serum and low-molecular-weight-iron in the liver of dietary iron-loaded rats. Int J Biochem. 1993 Feb;25(2):223–232. doi: 10.1016/0020-711x(93)90010-c. [DOI] [PubMed] [Google Scholar]
- Nielsen P., Heinrich H. C. Metabolism of iron from (3,5,5-trimethylhexanoyl)ferrocene in rats. A dietary model for severe iron overload. Biochem Pharmacol. 1993 Jan 26;45(2):385–391. doi: 10.1016/0006-2952(93)90074-7. [DOI] [PubMed] [Google Scholar]
- Pool T. B., Heitman T. O., Buck M. A. Changes in nuclear shape and mitochondrial structure do not accompany the loss of division potential in human fibroblasts in vitro. Am J Anat. 1981 Dec;162(4):369–382. doi: 10.1002/aja.1001620407. [DOI] [PubMed] [Google Scholar]
- Porter J. B., Abeysinghe R. D., Marshall L., Hider R. C., Singh S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood. 1996 Jul 15;88(2):705–713. [PubMed] [Google Scholar]
- Qian Z. M., Morgan E. H. Changes in the uptake of transferrin-free and transferrin-bound iron during reticulocyte maturation in vivo and in vitro. Biochim Biophys Acta. 1992 Apr 30;1135(1):35–43. doi: 10.1016/0167-4889(92)90163-6. [DOI] [PubMed] [Google Scholar]
- Serra R., Isom H. C. Stimulation of DNA synthesis and protooncogene expression in primary rat hepatocytes in long-term DMSO culture. J Cell Physiol. 1993 Mar;154(3):543–553. doi: 10.1002/jcp.1041540313. [DOI] [PubMed] [Google Scholar]
- Sims J. R., Karp S., Ingber D. E. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J Cell Sci. 1992 Dec;103(Pt 4):1215–1222. doi: 10.1242/jcs.103.4.1215. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stohs S. J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995 Feb;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Stonell L. M., Savigni D. L., Morgan E. H. Iron transport into erythroid cells by the Na+/Mg2+ antiport. Biochim Biophys Acta. 1996 Jun 13;1282(1):163–170. doi: 10.1016/0005-2736(96)00058-2. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Riedel H. D., Niederau C., Strohmeyer G. Pathogenesis of genetic haemochromatosis. Eur J Clin Invest. 1993 Jun;23(6):321–329. doi: 10.1111/j.1365-2362.1993.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Trinder D., Batey R. G., Morgan E. H., Baker E. Effect of cellular iron concentration on iron uptake by hepatocytes. Am J Physiol. 1990 Oct;259(4 Pt 1):G611–G617. doi: 10.1152/ajpgi.1990.259.4.G611. [DOI] [PubMed] [Google Scholar]
- Wang W. C., Ahmed N., Hanna M. Non-transferrin-bound iron in long-term transfusion in children with congenital anemias. J Pediatr. 1986 Apr;108(4):552–557. doi: 10.1016/s0022-3476(86)80832-0. [DOI] [PubMed] [Google Scholar]
- Woodworth C. D., Isom H. C. Regulation of albumin gene expression in a series of rat hepatocyte cell lines immortalized by simian virus 40 and maintained in chemically defined medium. Mol Cell Biol. 1987 Oct;7(10):3740–3748. doi: 10.1128/mcb.7.10.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Isom H. C. Transformation of differentiated rat hepatocytes with adenovirus and adenovirus DNA. J Virol. 1987 Nov;61(11):3570–3579. doi: 10.1128/jvi.61.11.3570-3579.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C., Secott T., Isom H. C. Transformation of rat hepatocytes by transfection with simian virus 40 DNA to yield proliferating differentiated cells. Cancer Res. 1986 Aug;46(8):4018–4026. [PubMed] [Google Scholar]
- Wright T. L., Brissot P., Ma W. L., Weisiger R. A. Characterization of non-transferrin-bound iron clearance by rat liver. J Biol Chem. 1986 Aug 15;261(23):10909–10914. [PubMed] [Google Scholar]
- Wydner K. S., Godyn J. J., Lee M. L., Sciorra L. J. A new approach to the computer-assisted quantitative analysis of nuclear shape. Mod Pathol. 1991 Mar;4(2):154–160. [PubMed] [Google Scholar]