Abstract
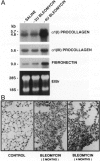
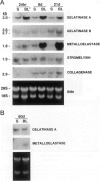
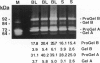
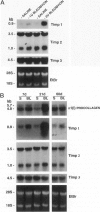
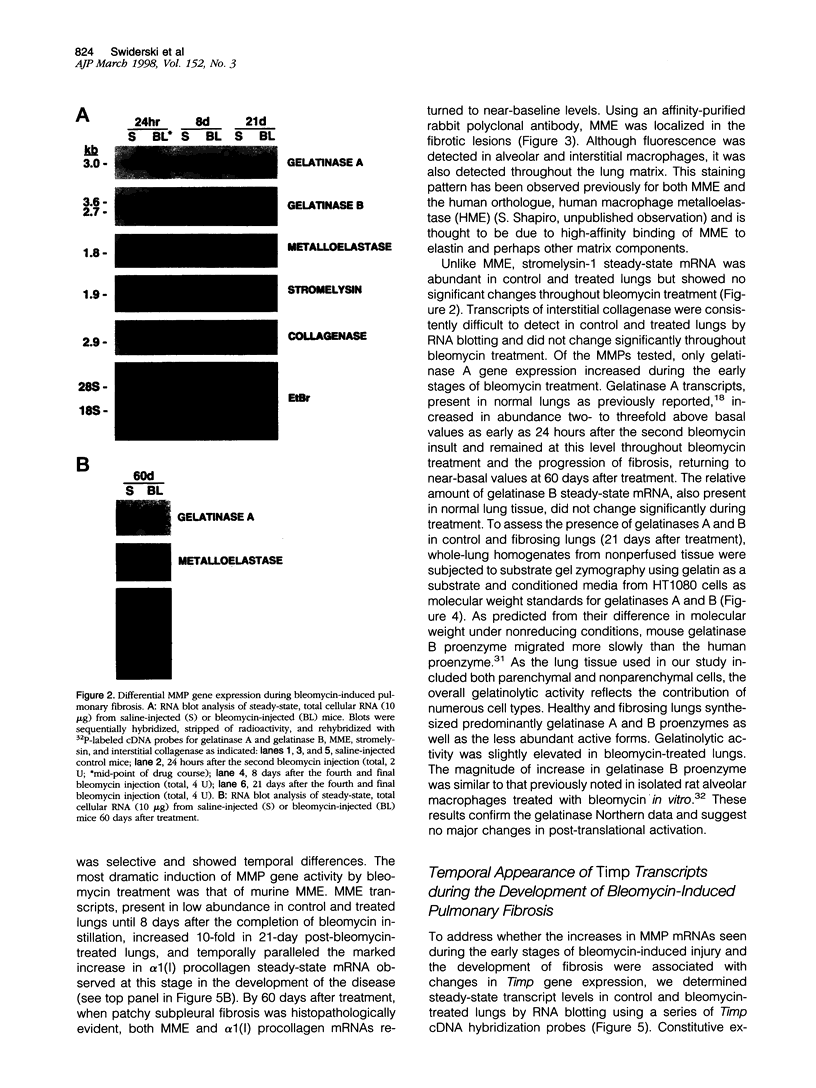
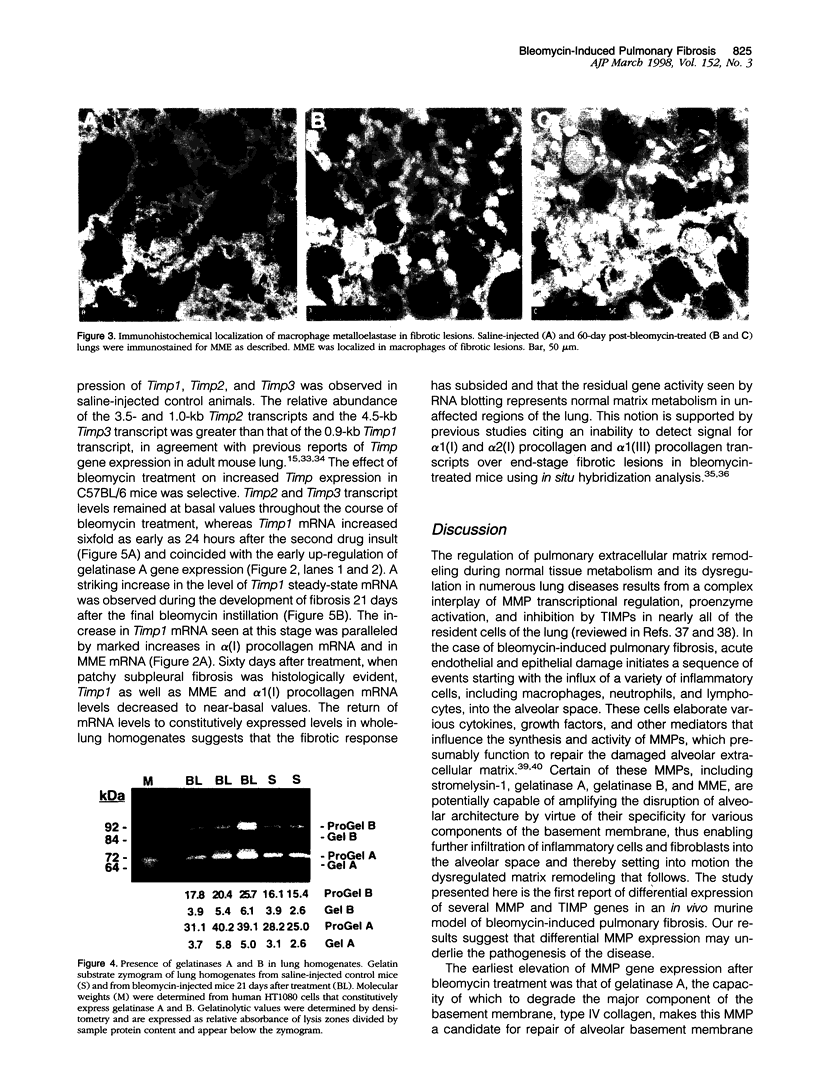
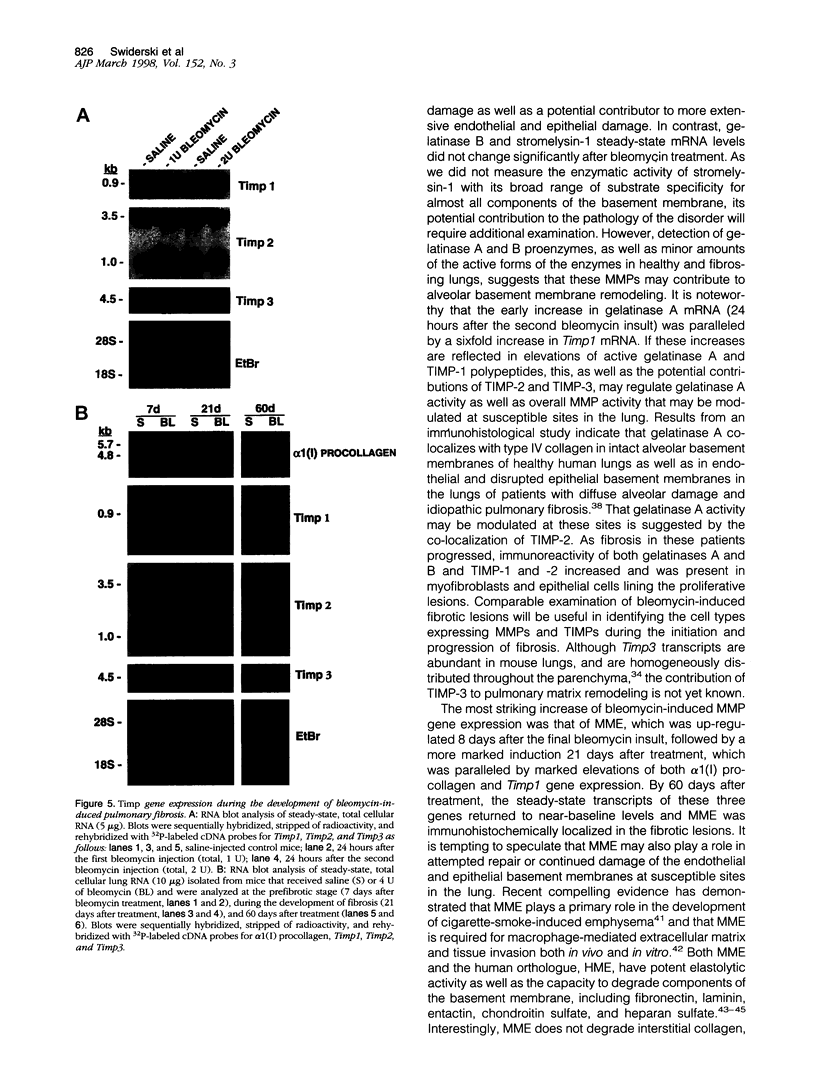
Exposure to the chemotherapeutic drug bleomycin leads to pulmonary fibrosis in humans and has been widely used in animal models of the disease. Using C57BL/6 bleomycin-sensitive mice, pulmonary fibrosis was induced by multiple intraperitoneal injections of the drug. An increase in the relative amounts of steady-state alpha1(I) procollagen, alpha1(III) procollagen, and fibronectin mRNA as well as histopathological evidence of fibrosis was observed. The effect of bleomycin on the expression of the enzymes responsible for extracellular matrix degradation, the matrix metalloproteinases (MMPs), and their inhibitors (TIMPs), was selective and showed temporal differences during the development of fibrosis. Of the MMPs tested, bleomycin treatment resulted in the up-regulation of gelatinase A and macrophage metalloelastase gene expression in whole-lung homogenates, whereas gelatinase B, stromelysin-1, and interstitial collagenase gene expression was not significantly changed. Timp2 and Timp3, the murine homologues of the respective TIMP genes, were constitutively expressed, whereas Timp1 was markedly up-regulated during fibrosis. The strong correlation between enhanced extracellular matrix gene expression, differential MMP and TIMP gene expression, and histopathological evidence of fibrosis suggest that dysregulated matrix remodeling is likely to contribute to the pathology of bleomycin-induced pulmonary fibrosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte S. S., Hayashi K., Seldin M. F., Mattei M. G., Hayashi M., Olsen B. R. Gene encoding a novel murine tissue inhibitor of metalloproteinases (TIMP), TIMP-3, is expressed in developing mouse epithelia, cartilage, and muscle, and is located on mouse chromosome 10. Dev Dyn. 1994 Jul;200(3):177–197. doi: 10.1002/aja.1002000302. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Werb Z. Mouse macrophage elastase. Purification and characterization as a metalloproteinase. Biochem J. 1981 Feb 1;193(2):589–605. doi: 10.1042/bj1930589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaaouaj A., Shipley J. M., Kobayashi D. K., Zimonjic D. B., Popescu N., Silverman G. A., Shapiro S. D. Human macrophage metalloelastase. Genomic organization, chromosomal location, gene linkage, and tissue-specific expression. J Biol Chem. 1995 Jun 16;270(24):14568–14575. doi: 10.1074/jbc.270.24.14568. [DOI] [PubMed] [Google Scholar]
- Benyon R. C., Iredale J. P., Goddard S., Winwood P. J., Arthur M. J. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 1996 Mar;110(3):821–831. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bowden D. H. Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest. 1984 May;50(5):487–488. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark S. D., Kobayashi D. K., Welgus H. G. Regulation of the expression of tissue inhibitor of metalloproteinases and collagenase by retinoids and glucocorticoids in human fibroblasts. J Clin Invest. 1987 Nov;80(5):1280–1288. doi: 10.1172/JCI113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Feng B., Edwards D. R., Cocuzzi E. T., Malyankar U. M. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993 Sep;59(3):329–341. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- Denholm E. M., Rollins S. M. Alveolar macrophage secretion of a 92-kDa gelatinase in response to bleomycin. Am J Physiol. 1993 Dec;265(6 Pt 1):L581–L585. doi: 10.1152/ajplung.1993.265.6.L581. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Beaudry P. P., Laing T. D., Kowal V., Leco K. J., Leco P. A., Lim M. S. The roles of tissue inhibitors of metalloproteinases in tissue remodelling and cell growth. Int J Obes Relat Metab Disord. 1996 Mar;20 (Suppl 3):S9–15. [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubert T., Johnston J., Berliner N. Cloning and expression of the cDNA encoding mouse neutrophil gelatinase: demonstration of coordinate secondary granule protein gene expression during terminal neutrophil maturation. Blood. 1993 Nov 15;82(10):3192–3197. [PubMed] [Google Scholar]
- Greene J., Wang M., Liu Y. E., Raymond L. A., Rosen C., Shi Y. E. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996 Nov 29;271(48):30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- Gronski T. J., Jr, Martin R. L., Kobayashi D. K., Walsh B. C., Holman M. C., Huber M., Van Wart H. E., Shapiro S. D. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem. 1997 May 2;272(18):12189–12194. doi: 10.1074/jbc.272.18.12189. [DOI] [PubMed] [Google Scholar]
- Harrison J. H., Jr, Lazo J. S. High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther. 1987 Dec;243(3):1185–1194. [PubMed] [Google Scholar]
- Hautamaki R. D., Kobayashi D. K., Senior R. M., Shapiro S. D. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997 Sep 26;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Hay J., Shahzeidi S., Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65(2):81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Stetler-Stevenson W. G., Fleming M. V., Fishback N., Koss M. N., Liotta L. A., Ferrans V. J., Travis W. D. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol. 1996 Oct;149(4):1241–1256. [PMC free article] [PubMed] [Google Scholar]
- Henriet P., Rousseau G. G., Eeckhout Y. Cloning and sequencing of mouse collagenase cDNA. Divergence of mouse and rat collagenases from the other mammalian collagenases. FEBS Lett. 1992 Sep 28;310(2):175–178. doi: 10.1016/0014-5793(92)81323-e. [DOI] [PubMed] [Google Scholar]
- Kato Y., Nakayama Y., Umeda M., Miyazaki K. Induction of 103-kDa gelatinase/type IV collagenase by acidic culture conditions in mouse metastatic melanoma cell lines. J Biol Chem. 1992 Jun 5;267(16):11424–11430. [PubMed] [Google Scholar]
- Kerr L. D., Olashaw N. E., Matrisian L. M. Transforming growth factor beta 1 and cAMP inhibit transcription of epidermal growth factor- and oncogene-induced transin RNA. J Biol Chem. 1988 Nov 15;263(32):16999–17005. [PubMed] [Google Scholar]
- Leco K. J., Apte S. S., Taniguchi G. T., Hawkes S. P., Khokha R., Schultz G. A., Edwards D. R. Murine tissue inhibitor of metalloproteinases-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett. 1997 Jan 20;401(2-3):213–217. doi: 10.1016/s0014-5793(96)01474-3. [DOI] [PubMed] [Google Scholar]
- Leco K. J., Hayden L. J., Sharma R. R., Rocheleau H., Greenberg A. H., Edwards D. R. Differential regulation of TIMP-1 and TIMP-2 mRNA expression in normal and Ha-ras-transformed murine fibroblasts. Gene. 1992 Aug 15;117(2):209–217. doi: 10.1016/0378-1119(92)90731-4. [DOI] [PubMed] [Google Scholar]
- Leco K. J., Khokha R., Pavloff N., Hawkes S. P., Edwards D. R. Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem. 1994 Mar 25;269(12):9352–9360. [PubMed] [Google Scholar]
- Matrisian L. M., Bowden G. T., Krieg P., Fürstenberger G., Briand J. P., Leroy P., Breathnach R. The mRNA coding for the secreted protease transin is expressed more abundantly in malignant than in benign tumors. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9413–9417. doi: 10.1073/pnas.83.24.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Metsäranta M., Toman D., De Crombrugghe B., Vuorio E. Specific hybridization probes for mouse type I, II, III and IX collagen mRNAs. Biochim Biophys Acta. 1991 Jun 13;1089(2):241–243. doi: 10.1016/0167-4781(91)90014-d. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Grappone C., Pellegrini G., Pinzani M., Casini A., Calabró A., Ciancio G., Stefanini F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994 Mar;144(3):528–537. [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Ebihara I., Osada S., Takahashi T., Yamamoto M., Tomino Y., Koide H. Gene expression of metalloproteinases and their inhibitor in renal tissue of New Zealand black/white F1 mice. Clin Sci (Lond) 1993 Sep;85(3):295–301. doi: 10.1042/cs0850295. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., FitzGerald M. X. Matrix metalloproteases and lung disease. Thorax. 1994 Jun;49(6):602–609. doi: 10.1136/thx.49.6.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Pardo A., Selman M., Ramírez R., Ramos C., Montaño M., Stricklin G., Raghu G. Production of collagenase and tissue inhibitor of metalloproteinases by fibroblasts derived from normal and fibrotic human lungs. Chest. 1992 Oct;102(4):1085–1089. doi: 10.1378/chest.102.4.1085. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992 Jan-Mar;18(1):29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Raghow R., Lurie S., Seyer J. M., Kang A. H. Profiles of steady state levels of messenger RNAs coding for type I procollagen, elastin, and fibronectin in hamster lungs undergoing bleomycin-induced interstitial pulmonary fibrosis. J Clin Invest. 1985 Nov;76(5):1733–1739. doi: 10.1172/JCI112163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen P., Sahlberg C., Huhtala P., Hurskainen T., Thesleff I., Tryggvason K. Molecular cloning of murine 72-kDa type IV collagenase and its expression during mouse development. J Biol Chem. 1992 Apr 15;267(11):7856–7862. [PubMed] [Google Scholar]
- Ries C., Petrides P. E. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe Seyler. 1995 Jun;376(6):345–355. [PubMed] [Google Scholar]
- Rosenberg G. A., Dencoff J. E., Correa N., Jr, Reiners M., Ford C. C. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood-brain barrier injury. Neurology. 1996 Jun;46(6):1626–1632. doi: 10.1212/wnl.46.6.1626. [DOI] [PubMed] [Google Scholar]
- Rossi G. A., Szapiel S., Ferrans V. J., Crystal R. G. Susceptibility to experimental interstitial lung disease is modified by immune- and non-immune-related genes. Am Rev Respir Dis. 1987 Feb;135(2):448–455. doi: 10.1164/arrd.1987.135.2.448. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Kunkel R. G., Phan S. H. The role of strain variation in murine bleomycin-induced pulmonary fibrosis. Am Rev Respir Dis. 1983 Jan;127(1):63–66. doi: 10.1164/arrd.1983.127.1.63. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shahzeidi S., Jeffery P. K., Laurent G. J., McAnulty R. J. Increased type I procollagen mRNA transcripts in the lungs of mice during the development of bleomycin-induced fibrosis. Eur Respir J. 1994 Nov;7(11):1938–1943. [PubMed] [Google Scholar]
- Shahzeidi S., Mulier B., de Crombrugghe B., Jeffery P. K., McAnulty R. J., Laurent G. J. Enhanced type III collagen gene expression during bleomycin induced lung fibrosis. Thorax. 1993 Jun;48(6):622–628. doi: 10.1136/thx.48.6.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. D., Griffin G. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Welgus H. G., Senior R. M., Ley T. J. Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J Biol Chem. 1992 Mar 5;267(7):4664–4671. [PubMed] [Google Scholar]
- Shapiro S. D., Kobayashi D. K., Ley T. J. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993 Nov 15;268(32):23824–23829. [PubMed] [Google Scholar]
- Shipley J. M., Wesselschmidt R. L., Kobayashi D. K., Ley T. J., Shapiro S. D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley T. D. New perspectives on basic mechanisms in lung disease. 6. Proteinase imbalance: its role in lung disease. Thorax. 1993 May;48(5):560–565. doi: 10.1136/thx.48.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant P., Menard O., Vignaud J. M., Martinet N., Martinet Y. The role of cytokines in human lung fibrosis. Monaldi Arch Chest Dis. 1996 Apr;51(2):145–152. [PubMed] [Google Scholar]
- Waterhouse P., Denhardt D. T., Khokha R. Temporal expression of tissue inhibitors of metalloproteinases in mouse reproductive tissues during gestation. Mol Reprod Dev. 1993 Jul;35(3):219–226. doi: 10.1002/mrd.1080350302. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]
- Woessner J. F., Jr The family of matrix metalloproteinases. Ann N Y Acad Sci. 1994 Sep 6;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- Zhang K., Gharaee-Kermani M., McGarry B., Phan S. H. In situ hybridization analysis of rat lung alpha 1(I) and alpha 2(I) collagen gene expression in pulmonary fibrosis induced by endotracheal bleomycin injection. Lab Invest. 1994 Feb;70(2):192–202. [PubMed] [Google Scholar]