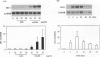Abstract
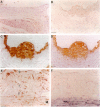
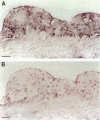
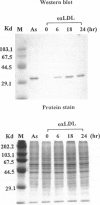
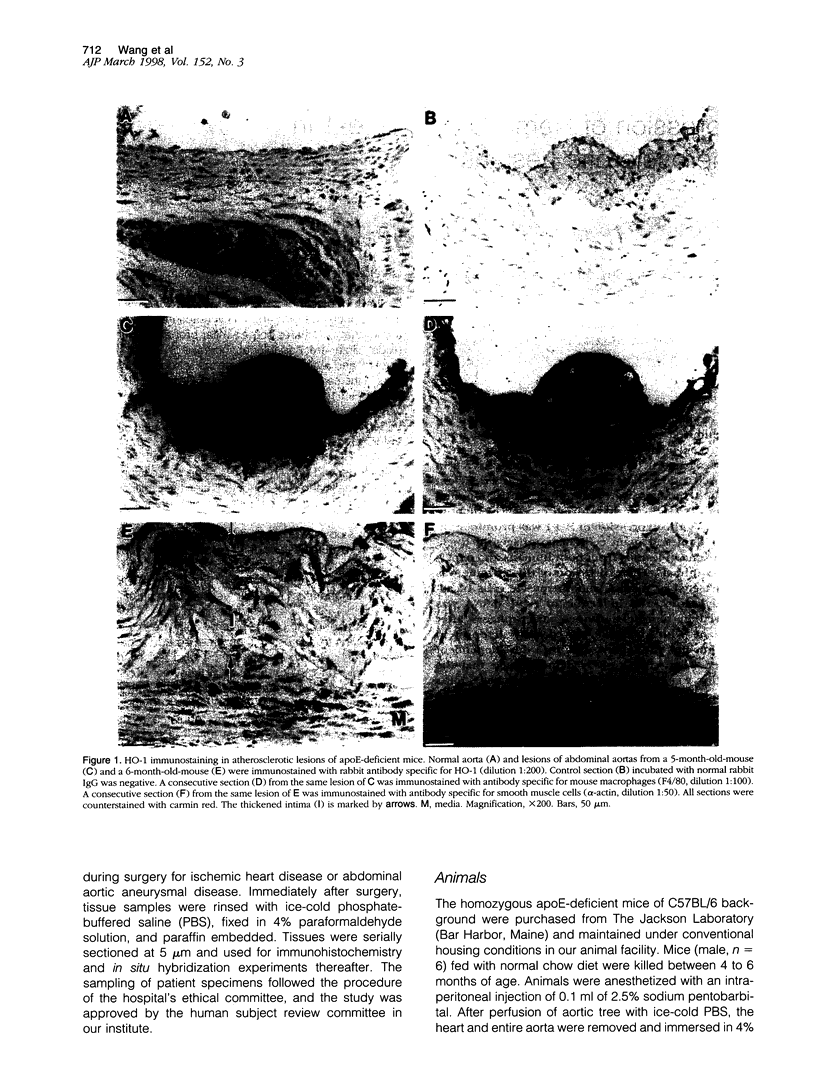
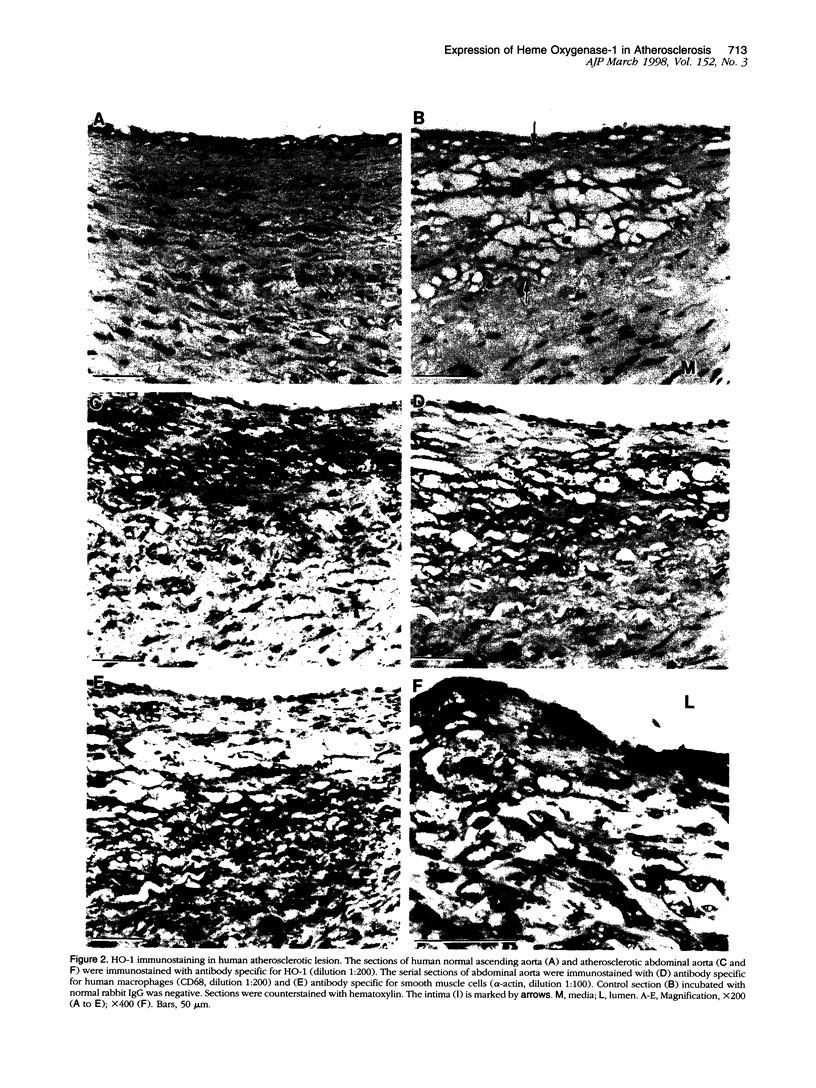
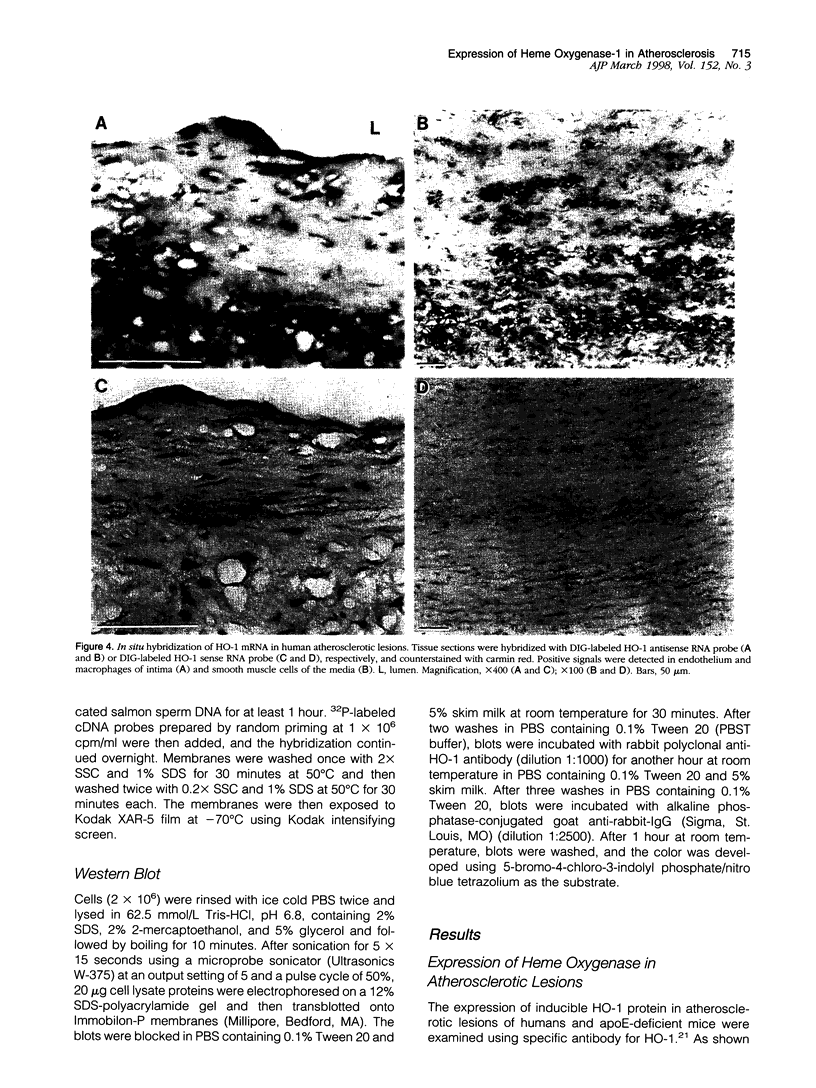
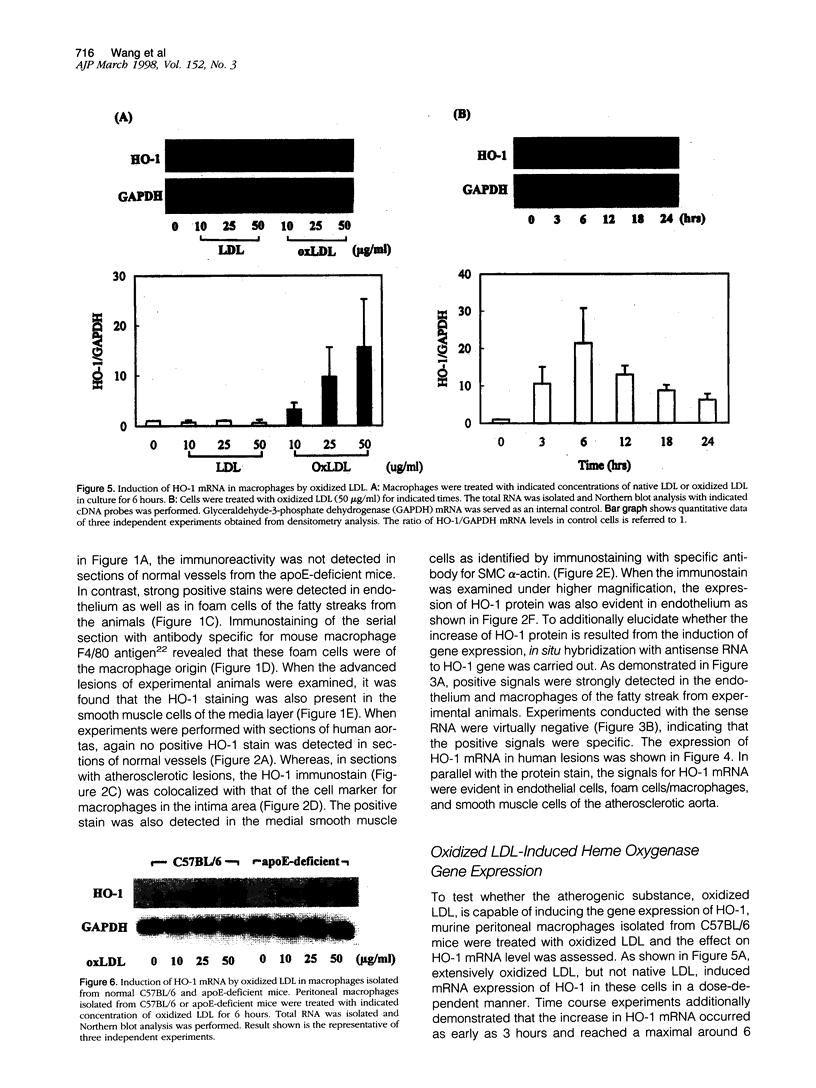
Heme oxygenase-1 (HO-1) is a heme-degradation enzyme induced under various oxidative stress conditions. To elucidate the potential involvement of HO-1 in atherogenesis, the expression of this enzyme in atherosclerotic lesions of apolipoprotein E-deficient mice and humans were examined. Both immunostaining and in situ hybridization clearly demonstrated that the expression of HO-1 was prominent in endothelium and foam cells/macrophages of thickened intima in lesions from both humans and experimental animals. The expression of this enzyme was also detected in medial smooth muscle cells of advanced lesions. The induction of HO-1 mRNA was observed in murine peritoneal macrophages after treatment with oxidized low density lipoprotein (LDL) but not with native LDL in a dose-dependent manner. Time course study demonstrated that the induction was prominent at 3 hours, reached a maximal induction at 6 hours, and remained evident up to 24 hours after oxidized LDL treatment. The degree of induction was in concordant with the extent of oxidation in the LDL preparation. Lysophosphatidylcholine, one of the major components present in oxidized LDL, was ineffective to induce the gene expression, suggesting that other lipophilic substances derived from LDL oxidation are responsible for the induction of HO-1. These results clearly demonstrate that HO-1 is one of the stress proteins expressed in atherosclerotic lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Lavrovsky Y., Schwartzman M. L., Stoltz R. A., Levere R. D., Gerritsen M. E., Shibahara S., Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6798–6802. doi: 10.1073/pnas.92.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Balla J., Balla G., Croatt A. J., Vercellotti G. M., Nath K. A. Renal tubular epithelial cells mimic endothelial cells upon exposure to oxidized LDL. Am J Physiol. 1996 Oct;271(4 Pt 2):F814–F823. doi: 10.1152/ajprenal.1996.271.4.F814. [DOI] [PubMed] [Google Scholar]
- Alam J. Multiple elements within the 5' distal enhancer of the mouse heme oxygenase-1 gene mediate induction by heavy metals. J Biol Chem. 1994 Oct 7;269(40):25049–25056. [PubMed] [Google Scholar]
- Alam J., Shibahara S., Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989 Apr 15;264(11):6371–6375. [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Balla J., Nath K. A., Balla G., Juckett M. B., Jacob H. S., Vercellotti G. M. Endothelial cell heme oxygenase and ferritin induction in rat lung by hemoglobin in vivo. Am J Physiol. 1995 Feb;268(2 Pt 1):L321–L327. doi: 10.1152/ajplung.1995.268.2.L321. [DOI] [PubMed] [Google Scholar]
- Berliner J. A., Navab M., Fogelman A. M., Frank J. S., Demer L. L., Edwards P. A., Watson A. D., Lusis A. J. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995 May 1;91(9):2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- Björkerud B., Björkerud S. Contrary effects of lightly and strongly oxidized LDL with potent promotion of growth versus apoptosis on arterial smooth muscle cells, macrophages, and fibroblasts. Arterioscler Thromb Vasc Biol. 1996 Mar;16(3):416–424. doi: 10.1161/01.atv.16.3.416. [DOI] [PubMed] [Google Scholar]
- Cantoni L., Rossi C., Rizzardini M., Gadina M., Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J. 1991 Nov 1;279(Pt 3):891–894. doi: 10.1042/bj2790891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y. C., Howe P. H., DiCorleto P. E., Chisolm G. M. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem. 1996 Jul 26;271(30):17791–17797. doi: 10.1074/jbc.271.30.17791. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cruse I., Maines M. D. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J Biol Chem. 1988 Mar 5;263(7):3348–3353. [PubMed] [Google Scholar]
- Dennery P. A., Sridhar K. J., Lee C. S., Wong H. E., Shokoohi V., Rodgers P. A., Spitz D. R. Heme oxygenase-mediated resistance to oxygen toxicity in hamster fibroblasts. J Biol Chem. 1997 Jun 6;272(23):14937–14942. doi: 10.1074/jbc.272.23.14937. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- Furchgott R. F., Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28(1-3):52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Zhuo M., Arancio O. Nitric oxide and carbon monoxide as possible retrograde messengers in hippocampal long-term potentiation. J Neurobiol. 1994 Jun;25(6):652–665. doi: 10.1002/neu.480250607. [DOI] [PubMed] [Google Scholar]
- Ishikawa K., Navab M., Leitinger N., Fogelman A. M., Lusis A. J. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997 Sep 1;100(5):1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya C., Domino E. F., Yang G. Y., Stern J. D., Betz A. L. Preischemic but not postischemic zinc protoporphyrin treatment reduces infarct size and edema accumulation after temporary focal cerebral ischemia in rats. Stroke. 1995 Jun;26(6):1035–1038. doi: 10.1161/01.str.26.6.1035. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Applegate L. A., Tromvoukis Y., Tyrrell R. M. Oxidant stress leads to transcriptional activation of the human heme oxygenase gene in cultured skin fibroblasts. Mol Cell Biol. 1990 Sep;10(9):4967–4969. doi: 10.1128/mcb.10.9.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Induction of the heme oxygenase gene in human skin fibroblasts by hydrogen peroxide and UVA (365 nm) radiation: evidence for the involvement of the hydroxyl radical. Carcinogenesis. 1990 May;11(5):787–791. doi: 10.1093/carcin/11.5.787. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y., Schwartzman M. L., Levere R. D., Kappas A., Abraham N. G. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz M. L., Hughes H., Mitchell J. R., Via D. P., Guyton J. R., Taylor A. A., Gotto A. M., Jr, Smith C. V. Lipid hydroperoxy and hydroxy derivatives in copper-catalyzed oxidation of low density lipoprotein. J Lipid Res. 1990 Jun;31(6):1043–1050. [PubMed] [Google Scholar]
- Llesuy S. F., Tomaro M. L. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994 Aug 11;1223(1):9–14. doi: 10.1016/0167-4889(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Zinc . protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim Biophys Acta. 1981 Mar 18;673(3):339–350. doi: 10.1016/0304-4165(81)90465-7. [DOI] [PubMed] [Google Scholar]
- Morita T., Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995 Dec;96(6):2676–2682. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Perrella M. A., Lee M. E., Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Raines E. W., Abraham J. A., Klagsbrun M., Ross R. Lysophosphatidylcholine upregulates the level of heparin-binding epidermal growth factor-like growth factor mRNA in human monocytes. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1069–1073. doi: 10.1073/pnas.91.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath K. A., Balla G., Vercellotti G. M., Balla J., Jacob H. S., Levitt M. D., Rosenberg M. E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992 Jul;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil J., Stocker R. Bilirubin attenuates radical-mediated damage to serum albumin. FEBS Lett. 1993 Oct 4;331(3):281–284. doi: 10.1016/0014-5793(93)80353-v. [DOI] [PubMed] [Google Scholar]
- Neuzil J., Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994 Jun 17;269(24):16712–16719. [PubMed] [Google Scholar]
- Nutter L. M., Sierra E. E., Ngo E. O. Heme oxygenase does not protect human cells against oxidant stress. J Lab Clin Med. 1994 Apr;123(4):506–514. [PubMed] [Google Scholar]
- Pang J. H., Jiang M. J., Chen Y. L., Wang F. W., Wang D. L., Chu S. H., Chau L. Y. Increased ferritin gene expression in atherosclerotic lesions. J Clin Invest. 1996 May 15;97(10):2204–2212. doi: 10.1172/JCI118661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon K. L., Dwyer B. E., Nishimura R. N., Wallis R. A. Neuroprotection against CA1 injury with metalloporphyrins. Neuroreport. 1996 Jan 31;7(2):662–666. doi: 10.1097/00001756-199601310-00067. [DOI] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Qiao J. H., Xie P. Z., Fishbein M. C., Kreuzer J., Drake T. A., Demer L. L., Lusis A. J. Pathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb. 1994 Sep;14(9):1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- Renier G., Skamene E., DeSanctis J. B., Radzioch D. High macrophage lipoprotein lipase expression and secretion are associated in inbred murine strains with susceptibility to atherosclerosis. Arterioscler Thromb. 1993 Feb;13(2):190–196. doi: 10.1161/01.atv.13.2.190. [DOI] [PubMed] [Google Scholar]
- Rizzardini M., Terao M., Falciani F., Cantoni L. Cytokine induction of haem oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the haem oxygenase gene. Biochem J. 1993 Mar 1;290(Pt 2):343–347. doi: 10.1042/bj2900343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sakai M., Miyazaki A., Hakamata H., Sasaki T., Yui S., Yamazaki M., Shichiri M., Horiuchi S. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J Biol Chem. 1994 Dec 16;269(50):31430–31435. [PubMed] [Google Scholar]
- Schmidt H. H. NO., CO and .OH. Endogenous soluble guanylyl cyclase-activating factors. FEBS Lett. 1992 Jul 27;307(1):102–107. doi: 10.1016/0014-5793(92)80910-9. [DOI] [PubMed] [Google Scholar]
- Siow R. C., Ishii T., Sato H., Taketani S., Leake D. S., Sweiry J. H., Pearson J. D., Bannai S., Mann G. E. Induction of the antioxidant stress proteins heme oxygenase-1 and MSP23 by stress agents and oxidised LDL in cultured vascular smooth muscle cells. FEBS Lett. 1995 Jul 17;368(2):239–242. doi: 10.1016/0014-5793(95)00650-x. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Kutty R. K., Richey P. L., Yan S. D., Stern D., Chader G. J., Wiggert B., Petersen R. B., Perry G. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. Am J Pathol. 1994 Jul;145(1):42–47. [PMC free article] [PubMed] [Google Scholar]
- Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9(2):101–112. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Vile G. F., Basu-Modak S., Waltner C., Tyrrell R. M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile G. F., Tyrrell R. M. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993 Jul 15;268(20):14678–14681. [PubMed] [Google Scholar]
- Witztum J. L. The oxidation hypothesis of atherosclerosis. Lancet. 1994 Sep 17;344(8925):793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- Wu T. W., Fung K. P., Wu J., Yang C. C., Weisel R. D. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996 Mar 22;51(6):859–862. doi: 10.1016/0006-2952(95)02395-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Horio F., Hashizume T., Tanaka M., Ikeda S., Kakinuma A., Nakajima H. Bilirubin is oxidized in rats treated with endotoxin and acts as a physiological antioxidant synergistically with ascorbic acid in vivo. Biochem Biophys Res Commun. 1995 Sep 5;214(1):11–19. doi: 10.1006/bbrc.1995.2250. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Terakado M., Horio F., Aoki K., Tanaka M., Nakajima H. Role of bilirubin as an antioxidant in an ischemia-reperfusion of rat liver and induction of heme oxygenase. Biochem Biophys Res Commun. 1996 Jun 5;223(1):129–135. doi: 10.1006/bbrc.1996.0857. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Biro P., Cohen T., Müller R. M., Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988 Feb 1;171(3):457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]