Abstract
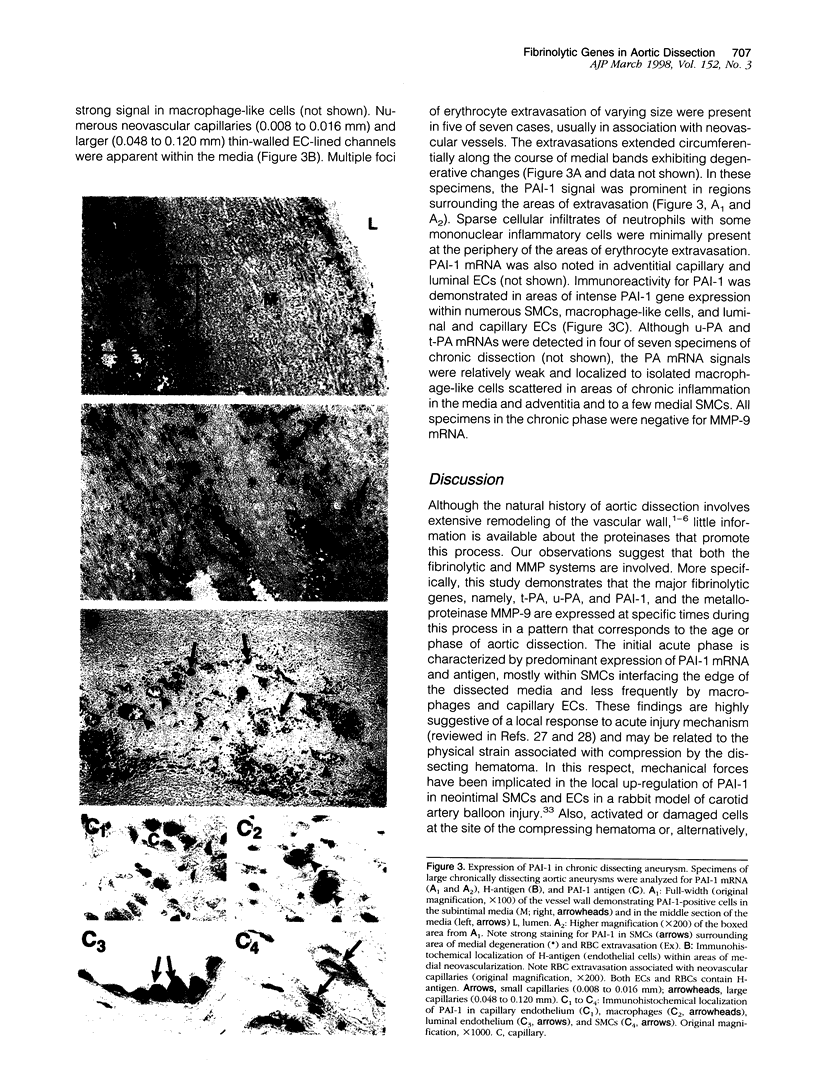
Although extensive tissue remodeling occurs during the various phases of aortic dissection, the underlying proteinases remain to be identified. Matrix metalloproteinase-9 (MMP-9) and components of the fibrinolytic system have been implicated in numerous tissue remodeling events and were therefore analyzed in surgical specimens of acute (n = 9), subacute (n = 4), and chronic (n = 7) aortic dissection by in situ hybridization. In the acute phase, intense plasminogen activator inhibitor 1 (PAI-1) gene expression was apparent in areas interfacing the dissecting hematoma, but no tissue-type PA (t-PA), urokinase-type PA (u-PA), or MMP-9 mRNAs were detected. Although PAI-1 mRNA was still present in the subacute phase, t-PA, u-PA, and MMP-9 mRNAs were now obvious, with PA gene expression co-localizing with areas of PAI-1 gene expression. In the chronic phase, PAI-1 mRNA was demonstrated around erythrocyte extravasations and surrounding bands of medial degeneration. However, there was little expression of PAs in these areas, and no MMP-9 was detected. Thus, fibrinolytic genes and MMP-9 are differentially expressed during the progression of aortic dissections. The kinetics of expression are consistent with acute fibrinolytic shutdown in response to the initial injury, a secondary subacute phase with active proteolysis, and finally, a chronic hypofibrinolytic state. Extensive neovascularization in the chronic phase may further reduce the physical stability of the dissected wall.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. L., Hibbs M. S., Kearney M., Loushin C., Isner J. M. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995 Apr 15;91(8):2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- Cambria R. P., Brewster D. C., Moncure A. C., Steinberg F. L., Abbott W. M. Spontaneous aortic dissection in the presence of coexistent or previously repaired atherosclerotic aortic aneurysm. Ann Surg. 1988 Nov;208(5):619–624. doi: 10.1097/00000658-198811000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Collen D. Gene targeting and gene transfer studies of the plasminogen/plasmin system: implications in thrombosis, hemostasis, neointima formation, and atherosclerosis. FASEB J. 1995 Jul;9(10):934–938. doi: 10.1096/fasebj.9.10.7615162. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Reidy M. A. Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest. 1986 Mar;54(3):295–303. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- DeSanctis R. W., Doroghazi R. M., Austen W. G., Buckley M. J. Aortic dissection. N Engl J Med. 1987 Oct 22;317(17):1060–1067. doi: 10.1056/NEJM198710223171705. [DOI] [PubMed] [Google Scholar]
- Freestone T., Turner R. J., Coady A., Higman D. J., Greenhalgh R. M., Powell J. T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995 Aug;15(8):1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. R., Liao S., Parks W. C., Thompson R. W. Medial neovascularization in abdominal aortic aneurysms: a histopathologic marker of aneurysmal degeneration with pathophysiologic implications. J Vasc Surg. 1995 May;21(5):761–772. doi: 10.1016/s0741-5214(05)80007-2. [DOI] [PubMed] [Google Scholar]
- James T. W., Wagner R., White L. A., Zwolak R. M., Brinckerhoff C. E. Induction of collagenase and stromelysin gene expression by mechanical injury in a vascular smooth muscle-derived cell line. J Cell Physiol. 1993 Nov;157(2):426–437. doi: 10.1002/jcp.1041570227. [DOI] [PubMed] [Google Scholar]
- Johnson M. D., Kim H. R., Chesler L., Tsao-Wu G., Bouck N., Polverini P. J. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J Cell Physiol. 1994 Jul;160(1):194–202. doi: 10.1002/jcp.1041600122. [DOI] [PubMed] [Google Scholar]
- Kamat B. R., Galli S. J., Barger A. C., Lainey L. L., Silverman K. J. Neovascularization and coronary atherosclerotic plaque: cinematographic localization and quantitative histologic analysis. Hum Pathol. 1987 Oct;18(10):1036–1042. doi: 10.1016/s0046-8177(87)80220-4. [DOI] [PubMed] [Google Scholar]
- Keeton M., Eguchi Y., Sawdey M., Ahn C., Loskutoff D. J. Cellular localization of type 1 plasminogen activator inhibitor messenger RNA and protein in murine renal tissue. Am J Pathol. 1993 Jan;142(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Lindsay J., Jr, Hurst J. W. Clinical features and prognosis in dissecting aneurysm of the aorta. A re-appraisal. Circulation. 1967 May;35(5):880–888. doi: 10.1161/01.cir.35.5.880. [DOI] [PubMed] [Google Scholar]
- Lupu F., Bergonzelli G. E., Heim D. A., Cousin E., Genton C. Y., Bachmann F., Kruithof E. K. Localization and production of plasminogen activator inhibitor-1 in human healthy and atherosclerotic arteries. Arterioscler Thromb. 1993 Jul;13(7):1090–1100. doi: 10.1161/01.atv.13.7.1090. [DOI] [PubMed] [Google Scholar]
- Lupu F., Heim D. A., Bachmann F., Hurni M., Kakkar V. V., Kruithof E. K. Plasminogen activator expression in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1995 Sep;15(9):1444–1455. doi: 10.1161/01.atv.15.9.1444. [DOI] [PubMed] [Google Scholar]
- Lusby R. J., Ferrell L. D., Ehrenfeld W. K., Stoney R. J., Wylie E. J. Carotid plaque hemorrhage. Its role in production of cerebral ischemia. Arch Surg. 1982 Nov;117(11):1479–1488. doi: 10.1001/archsurg.1982.01380350069010. [DOI] [PubMed] [Google Scholar]
- McMillan W. D., Patterson B. K., Keen R. R., Shively V. P., Cipollone M., Pearce W. H. In situ localization and quantification of mRNA for 92-kD type IV collagenase and its inhibitor in aneurysmal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol. 1995 Aug;15(8):1139–1144. doi: 10.1161/01.atv.15.8.1139. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lindenmayer A. E., Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens--evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol. 1994 Jan;7(1):82–90. [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny T., Sawdey M., Lawrence D., Millan J. L., Loskutoff D. J. Cloning and sequence of a cDNA coding for the human beta-migrating endothelial-cell-type plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6776–6780. doi: 10.1073/pnas.83.18.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podor T. J., Joshua P., Butcher M., Seiffert D., Loskutoff D., Gauldie J. Accumulation of type 1 plasminogen activator inhibitor and vitronectin at sites of cellular necrosis and inflammation. Ann N Y Acad Sci. 1992 Dec 4;667:173–177. doi: 10.1111/j.1749-6632.1992.tb51609.x. [DOI] [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Raghunath P. N., Tomaszewski J. E., Brady S. T., Caron R. J., Okada S. S., Barnathan E. S. Plasminogen activator system in human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1995 Sep;15(9):1432–1443. doi: 10.1161/01.atv.15.9.1432. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., McFall R. C. Platelet-derived growth factor and transforming growth factor-beta regulate plasminogen activator inhibitor-1 synthesis in vascular smooth muscle cells. J Biol Chem. 1991 May 25;266(15):9419–9427. [PubMed] [Google Scholar]
- Reilly J. M., Sicard G. A., Lucore C. L. Abnormal expression of plasminogen activators in aortic aneurysmal and occlusive disease. J Vasc Surg. 1994 May;19(5):865–872. doi: 10.1016/s0741-5214(94)70012-5. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Sawa H., Fujii S., Sobel B. E. Augmented arterial wall expression of type-1 plasminogen activator inhibitor induced by thrombosis. Arterioscler Thromb. 1992 Dec;12(12):1507–1515. doi: 10.1161/01.atv.12.12.1507. [DOI] [PubMed] [Google Scholar]
- Sawa H., Lundgren C., Sobel B. E., Fujii S. Increased intramural expression of plasminogen activator inhibitor type 1 after balloon injury: a potential progenitor of restenosis. J Am Coll Cardiol. 1994 Dec;24(7):1742–1748. doi: 10.1016/0735-1097(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Sawdey M. S., Loskutoff D. J. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest. 1991 Oct;88(4):1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatmann T. J., Becker A. E. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977 Jan;39(1):13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- Schlatmann T. J., Becker A. E. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977 Jan;39(1):21–26. doi: 10.1016/s0002-9149(77)80005-2. [DOI] [PubMed] [Google Scholar]
- Schneiderman J., Bordin G. M., Engelberg I., Adar R., Seiffert D., Thinnes T., Bernstein E. F., Dilley R. B., Loskutoff D. J. Expression of fibrinolytic genes in atherosclerotic abdominal aortic aneurysm wall. A possible mechanism for aneurysm expansion. J Clin Invest. 1995 Jul;96(1):639–645. doi: 10.1172/JCI118079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman J., Sawdey M. S., Keeton M. R., Bordin G. M., Bernstein E. F., Dilley R. B., Loskutoff D. J. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman J., Sawdey M., Craig H., Thinnes T., Bordin G., Loskutoff D. J. Type 1 plasminogen activator inhibitor gene expression following partial hepatectomy. Am J Pathol. 1993 Sep;143(3):753–762. [PMC free article] [PubMed] [Google Scholar]
- Shah P. K., Falk E., Badimon J. J., Fernandez-Ortiz A., Mailhac A., Villareal-Levy G., Fallon J. T., Regnstrom J., Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995 Sep 15;92(6):1565–1569. [PubMed] [Google Scholar]
- Slivka S. R., Loskutoff D. J. Platelets stimulate endothelial cells to synthesize type 1 plasminogen activator inhibitor. Evaluation of the role of transforming growth factor beta. Blood. 1991 Mar 1;77(5):1013–1019. [PubMed] [Google Scholar]
- Strauss B. H., Robinson R., Batchelor W. B., Chisholm R. J., Ravi G., Natarajan M. K., Logan R. A., Mehta S. R., Levy D. E., Ezrin A. M. In vivo collagen turnover following experimental balloon angioplasty injury and the role of matrix metalloproteinases. Circ Res. 1996 Sep;79(3):541–550. doi: 10.1161/01.res.79.3.541. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Swanson S. J., Clinton S. K., Ganz P., Cybulsky M. I., Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993 Oct;88(4 Pt 1):1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- Thompson R. W., Holmes D. R., Mertens R. A., Liao S., Botney M. D., Mecham R. P., Welgus H. G., Parks W. C. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995 Jul;96(1):318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. K., Hutchins G. M. Aortic dissecting aneurysms: causative factors in 204 subjects. Arch Pathol Lab Med. 1982 Apr;106(4):175–180. [PubMed] [Google Scholar]
- Wojta J., Gallicchio M., Zoellner H., Hufnagl P., Last K., Filonzi E. L., Binder B. R., Hamilton J. A., McGrath K. Thrombin stimulates expression of tissue-type plasminogen activator and plasminogen activator inhibitor type 1 in cultured human vascular smooth muscle cells. Thromb Haemost. 1993 Sep 1;70(3):469–474. [PubMed] [Google Scholar]
- Zempo N., Koyama N., Kenagy R. D., Lea H. J., Clowes A. W. Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol. 1996 Jan;16(1):28–33. doi: 10.1161/01.atv.16.1.28. [DOI] [PubMed] [Google Scholar]