Abstract
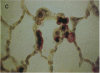
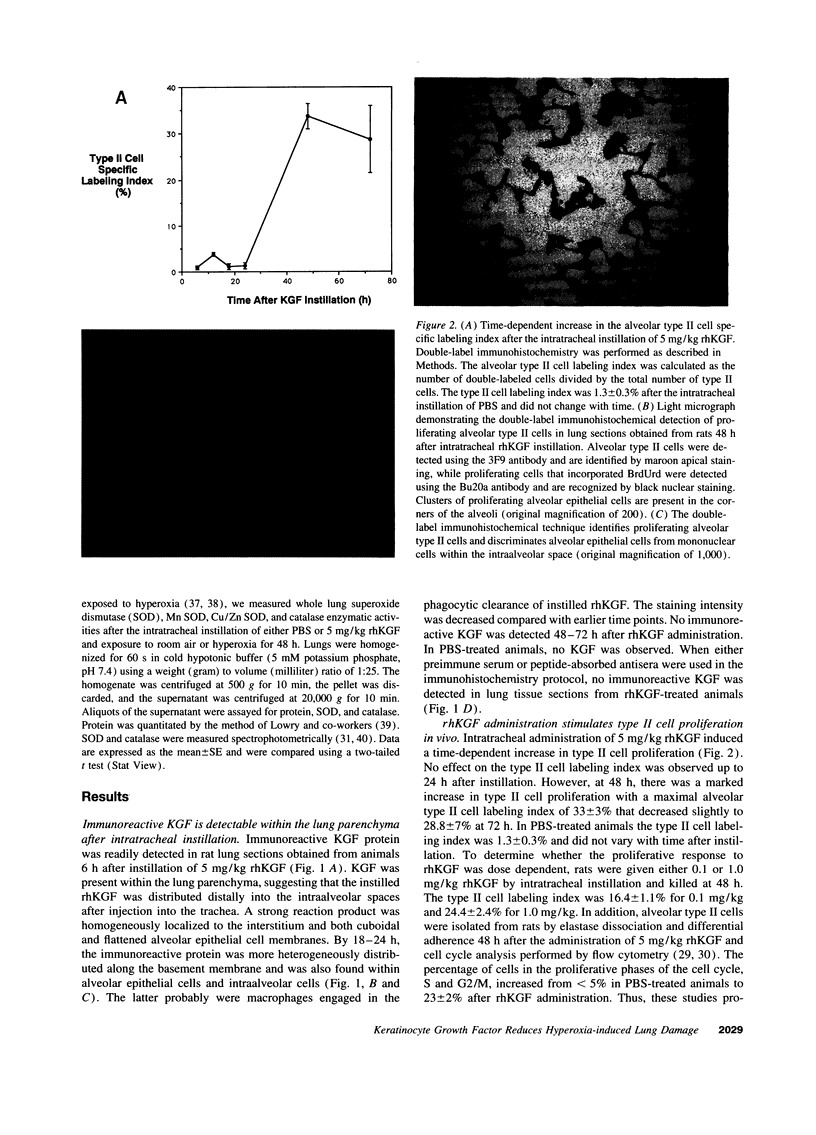
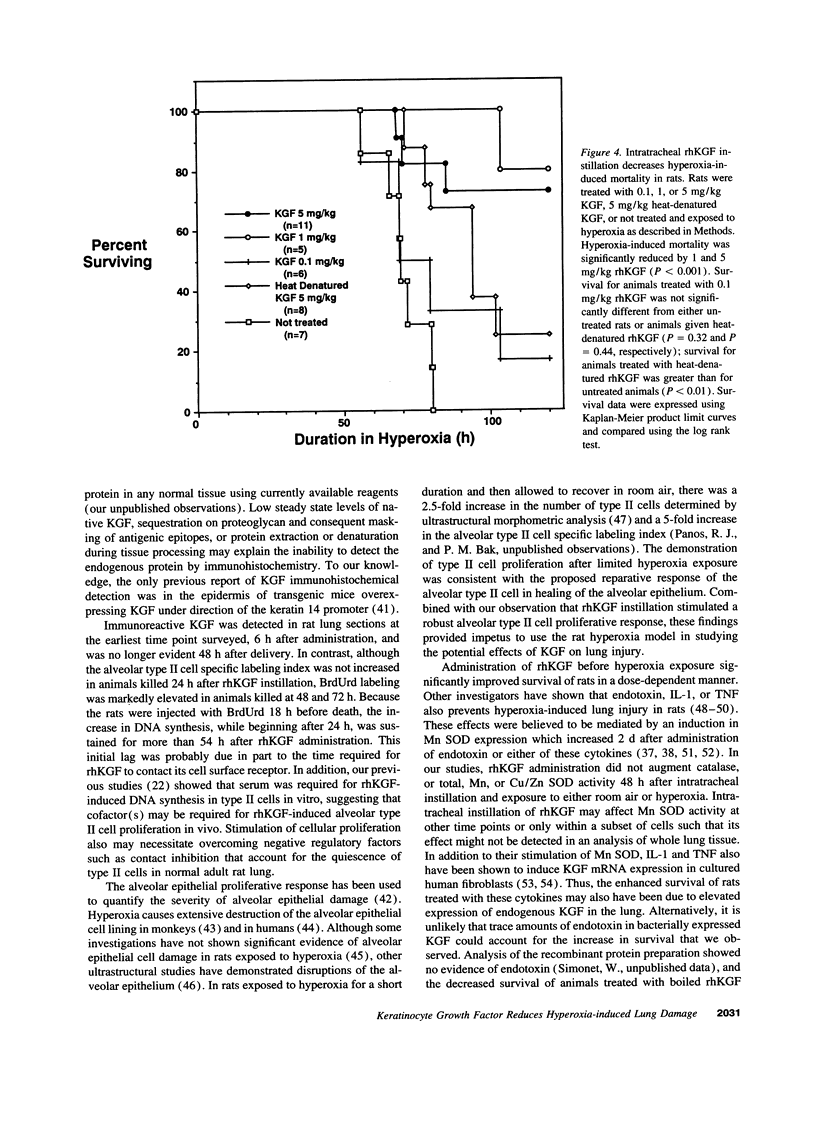
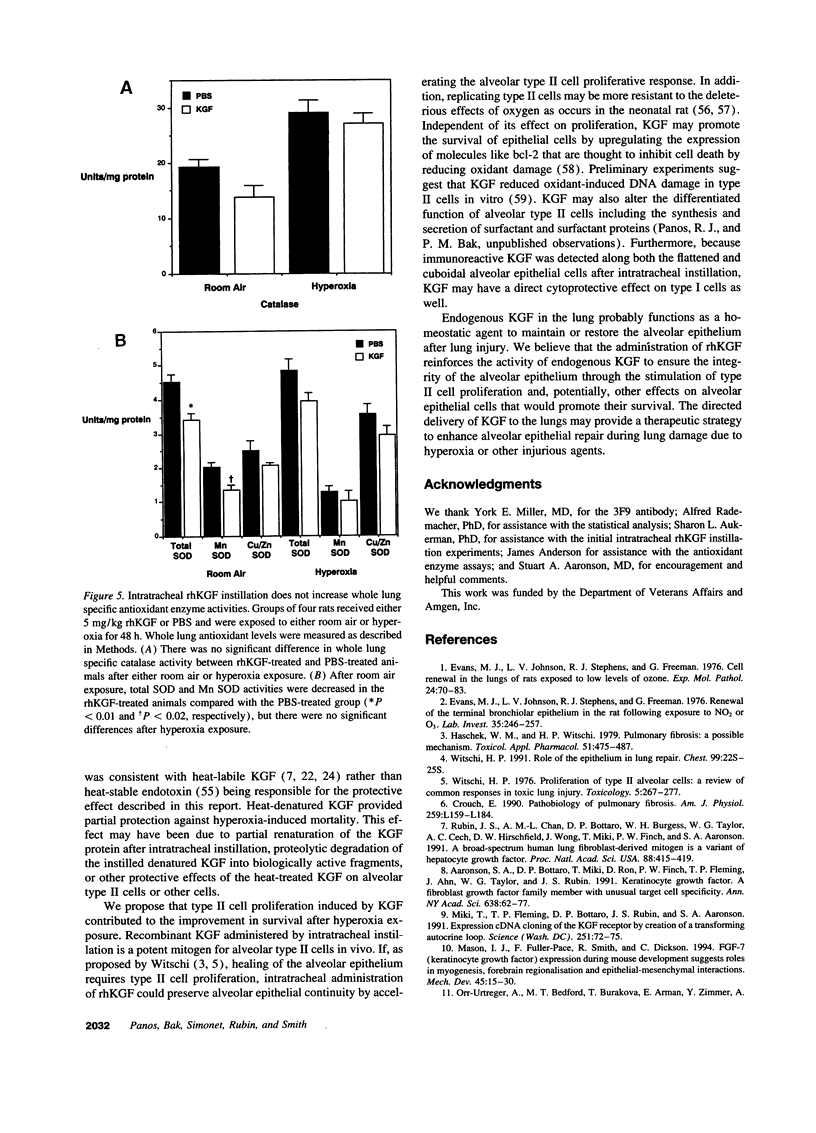
Alveolar type II cell proliferation occurs after many forms of lung injury and is thought to play a critical role in alveolar epithelial repair. Keratinocyte growth factor/fibroblast growth factor 7 (KGF) has been shown to promote alveolar type II cell growth in primary culture and alveolar epithelial hyperplasia in vivo. In this study, we used immunohistochemical analysis to determine the intrapulmonary distribution and cellular localization of recombinant human KGF (rhKGF) instilled into the trachea of rats. 6 h after administration, immunoreactive KGF was observed within the lung parenchyma and along alveolar epithelial cell membranes. By 18-24 h, KGF was detected intracellularly in alveolar epithelial cells and intraalveolar macrophages. Immunoreactive KGF was not demonstrable 48 h after delivery or in lung sections from PBS-treated animals. Intratracheal instillation of 5 mg/kg rhKGF stimulated a marked, time-dependent increase in the alveolar type II cell specific labeling index to a maximum level of 33 +/- 3% 48 h after rhKGF administration compared with 1.3 +/- 0.3% after PBS instillation. In addition, this increase in type II cell proliferation in vivo was documented by flow cytometric analysis of isolated type II cells which revealed a nearly fivefold increase in the proportion of cells traversing through the S and G2/M phases of the cell cycle. To test the hypothesis that KGFs effects on type II cells in vivo might affect the response to lung injury, rats were treated with rhKGF and exposed to hyperoxia. Animals that received 1 or 5 mg/kg rhKGF exhibited dramatically reduced mortality (P < 0.001, for both doses). Survival for animals treated with 0.1 mg/kg rhKGF was not significantly different from either untreated rats or animals treated with heat-denatured rhKGF. The lungs of rhKGF-treated animals that survived hyperoxia exposure had minimal hemorrhage and no exudate within the intraalveolar space. These experiments established that intratracheal administration of rhKGF stimulated alveolar type II cell proliferation in vivo and reduced hyperoxia-induced lung injury in rats. Directed delivery of KGF to the lungs may provide a therapeutic strategy to preserve or restore the alveolar epithelium during exposure to hyperoxia or other injurious agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Bottaro D. P., Miki T., Ron D., Finch P. W., Fleming T. P., Ahn J., Taylor W. G., Rubin J. S. Keratinocyte growth factor. A fibroblast growth factor family member with unusual target cell specificity. Ann N Y Acad Sci. 1991;638:62–77. doi: 10.1111/j.1749-6632.1991.tb49018.x. [DOI] [PubMed] [Google Scholar]
- Alarid E. T., Rubin J. S., Young P., Chedid M., Ron D., Aaronson S. A., Cunha G. R. Keratinocyte growth factor functions in epithelial induction during seminal vesicle development. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1074–1078. doi: 10.1073/pnas.91.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauchle M., Angermeyer K., Hübner G., Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994 Nov;9(11):3199–3204. [PubMed] [Google Scholar]
- Chedid M., Rubin J. S., Csaky K. G., Aaronson S. A. Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem. 1994 Apr 8;269(14):10753–10757. [PubMed] [Google Scholar]
- Chen B. L., Arakawa T., Morris C. F., Kenney W. C., Wells C. M., Pitt C. G. Aggregation pathway of recombinant human keratinocyte growth factor and its stabilization. Pharm Res. 1994 Nov;11(11):1581–1587. doi: 10.1023/a:1018905720139. [DOI] [PubMed] [Google Scholar]
- Clerch L. B., Massaro D. Tolerance of rats to hyperoxia. Lung antioxidant enzyme gene expression. J Clin Invest. 1993 Feb;91(2):499–508. doi: 10.1172/JCI116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol. 1990 Oct;259(4 Pt 1):L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G. Isolation and culture of alveolar type II cells. Am J Physiol. 1990 Apr;258(4 Pt 1):L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Dekker N. P., Cabral-Anderson L. J., Freeman G. Quantitation of damage to the alveolar epithelium by means of type 2 cell proliferation. Am Rev Respir Dis. 1978 Oct;118(4):787–790. doi: 10.1164/arrd.1978.118.4.787. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Johnson L. V., Stephens R. J., Freeman G. Cell renewal in the lungs of rats exposed to low levels of ozone. Exp Mol Pathol. 1976 Feb;24(1):70–83. doi: 10.1016/0014-4800(76)90058-7. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Johnson L. V., Stephens R. J., Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976 Sep;35(3):246–257. [PubMed] [Google Scholar]
- Finch P. W., Cunha G. R., Rubin J. S., Wong J., Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995 Jun;203(2):223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Tosco R., Wheelis R. F., Gould N. S., Kapanci Y. Oxygen pneumonitis in man. Ultrastructural observations on the development of alveolar lesions. Lab Invest. 1972 May;26(5):499–508. [PubMed] [Google Scholar]
- Guo L., Yu Q. C., Fuchs E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993 Mar;12(3):973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschek W. M., Witschi H. Pulmonary fibrosis--a possible mechanism. Toxicol Appl Pharmacol. 1979 Dec;51(3):475–487. doi: 10.1016/0041-008x(79)90372-7. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Housley R. M., Morris C. F., Boyle W., Ring B., Biltz R., Tarpley J. E., Aukerman S. L., Devine P. L., Whitehead R. H., Pierce G. F. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994 Nov;94(5):1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kauffman S. L., Burri P. H., Weibel E. R. The postnatal growth of the rat lung. II. Autoradiography. Anat Rec. 1974 Sep;180(1):63–76. doi: 10.1002/ar.1091800108. [DOI] [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mason I. J., Fuller-Pace F., Smith R., Dickson C. FGF-7 (keratinocyte growth factor) expression during mouse development suggests roles in myogenesis, forebrain regionalisation and epithelial-mesenchymal interactions. Mech Dev. 1994 Jan;45(1):15–30. doi: 10.1016/0925-4773(94)90050-7. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Merchenthaler I., Stankovics J., Gallyas F. A highly sensitive one-step method for silver intensification of the nickel-diaminobenzidine endproduct of peroxidase reaction. J Histochem Cytochem. 1989 Oct;37(10):1563–1565. doi: 10.1177/37.10.2674275. [DOI] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Bottaro D. P., Rubin J. S., Ron D., Aaronson S. A. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991 Jan 4;251(4989):72–75. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- Miller Y. E., Walker S. R., Spencer J. S., Kubo R. T., Mason R. J. Monoclonal antibodies specific for antigens expressed by rat type II alveolar epithelial and nonciliated bronchiolar cells. Exp Lung Res. 1989 Jul;15(4):635–649. doi: 10.3109/01902148909069623. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A., Bedford M. T., Burakova T., Arman E., Zimmer Y., Yayon A., Givol D., Lonai P. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2). Dev Biol. 1993 Aug;158(2):475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- Panos R. J., Mason R. J. Hypertrophic alveolar type II cells isolated after silica-induced lung injury are progressing through the cell cycle and maintain a commitment to DNA synthesis in primary culture. Chest. 1991 Mar;99(3 Suppl):27S–28S. doi: 10.1378/chest.99.3_supplement.27s. [DOI] [PubMed] [Google Scholar]
- Panos R. J., Rubin J. S., Csaky K. G., Aaronson S. A., Mason R. J. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993 Aug;92(2):969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panos R. J., Suwabe A., Leslie C. C., Mason R. J. Hypertrophic alveolar type II cells from silica-treated rats are committed to DNA synthesis in vitro. Am J Respir Cell Mol Biol. 1990 Jul;3(1):51–59. doi: 10.1165/ajrcmb/3.1.51. [DOI] [PubMed] [Google Scholar]
- Peters K., Werner S., Liao X., Wert S., Whitsett J., Williams L. Targeted expression of a dominant negative FGF receptor blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO J. 1994 Jul 15;13(14):3296–3301. doi: 10.1002/j.1460-2075.1994.tb06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Yanagihara D., Klopchin K., Danilenko D. M., Hsu E., Kenney W. C., Morris C. F. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994 Mar 1;179(3):831–840. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Bottaro D. P., Finch P. W., Morris D., Rubin J. S., Aaronson S. A. Expression of biologically active recombinant keratinocyte growth factor. Structure/function analysis of amino-terminal truncation mutants. J Biol Chem. 1993 Feb 5;268(4):2984–2988. [PubMed] [Google Scholar]
- Rubin J. S., Chan A. M., Bottaro D. P., Burgess W. H., Taylor W. G., Cech A. C., Hirschfield D. W., Wong J., Miki T., Finch P. W. A broad-spectrum human lung fibroblast-derived mitogen is a variant of hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):415–419. doi: 10.1073/pnas.88.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. J., Anderson J., Shamsuddin M., Hsueh W. Effect of fasting on hyperoxic lung injury in mice. The role of glutathione. Am Rev Respir Dis. 1990 Jan;141(1):141–149. doi: 10.1164/ajrccm/141.1.141. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Friedman H., Anderson J. Hyperoxic lung injury in mice: effect of neutrophil depletion and food deprivation. J Lab Clin Med. 1988 Apr;111(4):449–458. [PubMed] [Google Scholar]
- Smith L. J. Hyperoxic lung injury: biochemical, cellular, and morphologic characterization in the mouse. J Lab Clin Med. 1985 Sep;106(3):269–278. [PubMed] [Google Scholar]
- Smith L. J., Shamsuddin M., Anderson J., Hsueh W. Hyperoxic lung damage in mice: appearance and bioconversion of peptide leukotrienes. J Appl Physiol (1985) 1988 Mar;64(3):944–951. doi: 10.1152/jappl.1988.64.3.944. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Sommers E., Hunt C. E., Pachman L. Hyperoxic lung injury in mice: a possible protective role for prostacyclin. J Lab Clin Med. 1986 Nov;108(5):479–488. [PubMed] [Google Scholar]
- Staiano-Coico L., Krueger J. G., Rubin J. S., D'limi S., Vallat V. P., Valentino L., Fahey T., 3rd, Hawes A., Kingston G., Madden M. R. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993 Sep 1;178(3):865–878. doi: 10.1084/jem.178.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Berg J. T., White J. E., Lumb P. D., Lee C. Y., Tsan M. F. Protection against oxygen toxicity by tracheal insufflation of endotoxin: role of Mn SOD and alveolar macrophages. Am J Physiol. 1994 Jan;266(1 Pt 1):L38–L45. doi: 10.1152/ajplung.1994.266.1.L38. [DOI] [PubMed] [Google Scholar]
- Thet L. A., Parra S. C., Shelburne J. D. Sequential changes in lung morphology during the repair of acute oxygen-induced lung injury in adult rats. Exp Lung Res. 1986;11(3):209–228. doi: 10.3109/01902148609064297. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Lee C. Y., White J. E. Interleukin 1 protects rats against oxygen toxicity. J Appl Physiol (1985) 1991 Aug;71(2):688–697. doi: 10.1152/jappl.1991.71.2.688. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E. Kinetics of pulmonary superoxide dismutase in interleukin-1-induced oxygen-tolerant rats. Am J Physiol. 1992 Sep;263(3 Pt 1):L342–L347. doi: 10.1152/ajplung.1992.263.3.L342. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Santana T. A., Lee C. Y. Tracheal insufflation of tumor necrosis factor protects rats against oxygen toxicity. J Appl Physiol (1985) 1990 Mar;68(3):1211–1219. doi: 10.1152/jappl.1990.68.3.1211. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Treanor C., Shaffer J. B. Molecular basis for tumor necrosis factor-induced increase in pulmonary superoxide dismutase activities. Am J Physiol. 1990 Dec;259(6 Pt 1):L506–L512. doi: 10.1152/ajplung.1990.259.6.L506. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Cardiff R., Yin S., Bikhazi N., Biltz R., Morris C. F., Pierce G. F. Keratinocyte growth factor is a growth factor for mammary epithelium in vivo. The mammary epithelium of lactating rats is resistant to the proliferative action of keratinocyte growth factor. Am J Pathol. 1994 May;144(5):862–868. [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Longmuir K., Yin S., Biltz R., Morris C. F., Housley R. M., Pierce G. F. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994 Mar;93(3):1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Peters K. G., Longaker M. T., Fuller-Pace F., Banda M. J., Williams L. T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Smola H., Liao X., Longaker M. T., Krieg T., Hofschneider P. H., Williams L. T. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994 Nov 4;266(5186):819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- White C. W., Ghezzi P., Dinarello C. A., Caldwell S. A., McMurtry I. F., Repine J. E. Recombinant tumor necrosis factor/cachectin and interleukin 1 pretreatment decreases lung oxidized glutathione accumulation, lung injury, and mortality in rats exposed to hyperoxia. J Clin Invest. 1987 Jun;79(6):1868–1873. doi: 10.1172/JCI113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch I. H. Proliferation of type II alveolar cells: a review of common responses in toxic lung injury. Toxicology. 1976 Mar;5(3):267–277. doi: 10.1016/0300-483x(76)90046-9. [DOI] [PubMed] [Google Scholar]
- Witschi H. Role of the epithelium in lung repair. Chest. 1991 Mar;99(3 Suppl):22S–25S. doi: 10.1378/chest.99.3_supplement.22s. [DOI] [PubMed] [Google Scholar]
- Yi E. S., Yin S., Harclerode D. L., Bedoya A., Bikhazi N. B., Housley R. M., Aukerman S. L., Morris C. F., Pierce G. F., Ulich T. R. Keratinocyte growth factor induces pancreatic ductal epithelial proliferation. Am J Pathol. 1994 Jul;145(1):80–85. [PMC free article] [PubMed] [Google Scholar]