Abstract
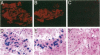
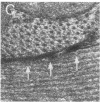
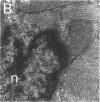
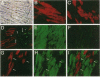
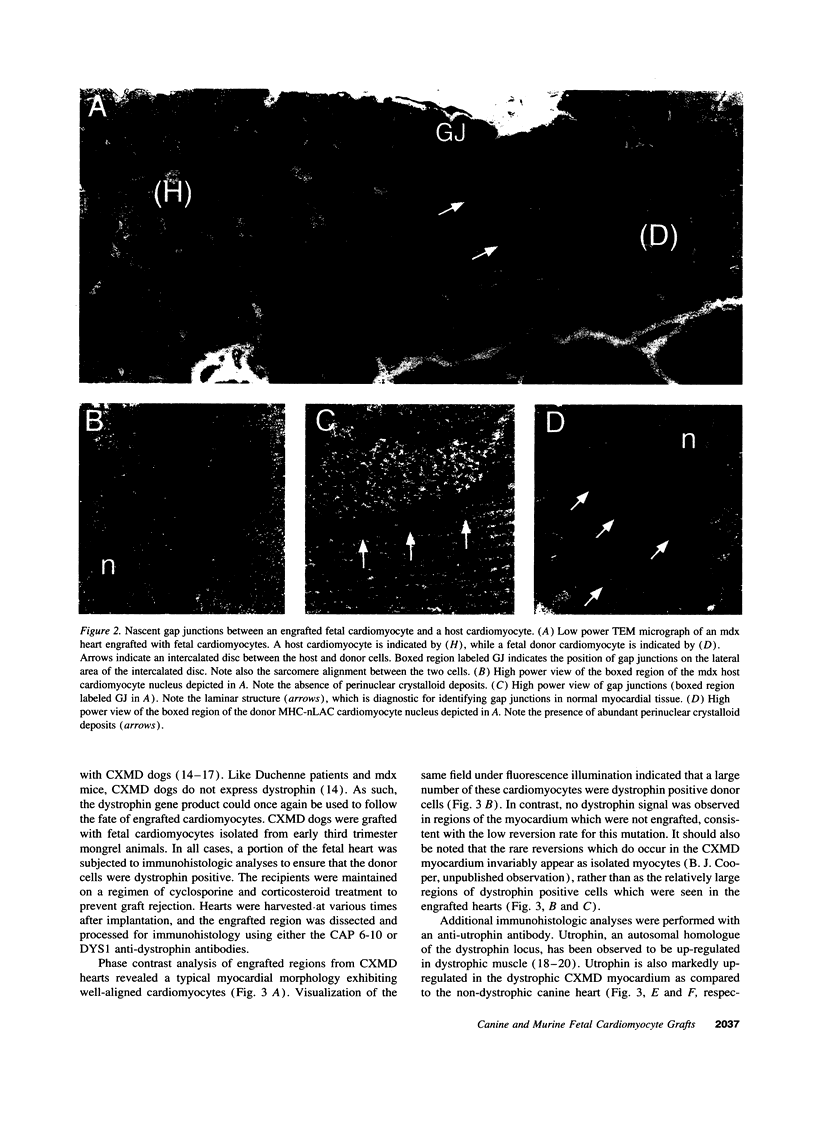
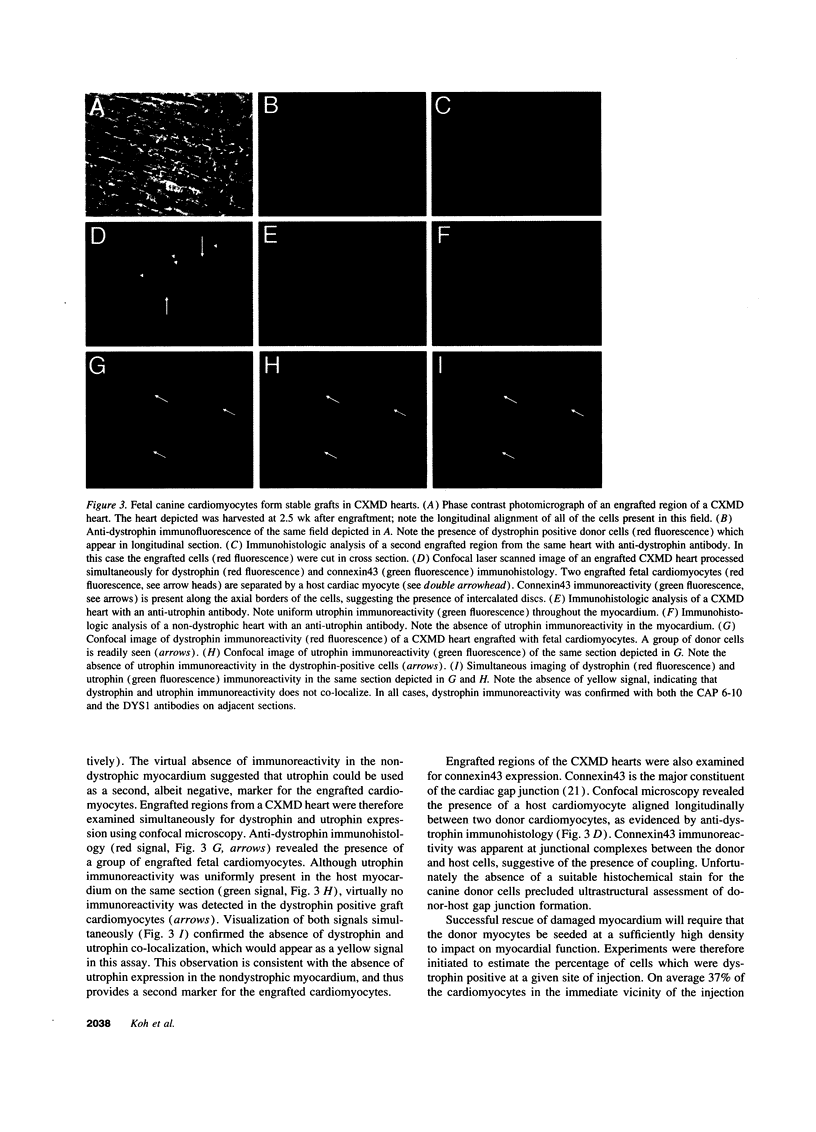
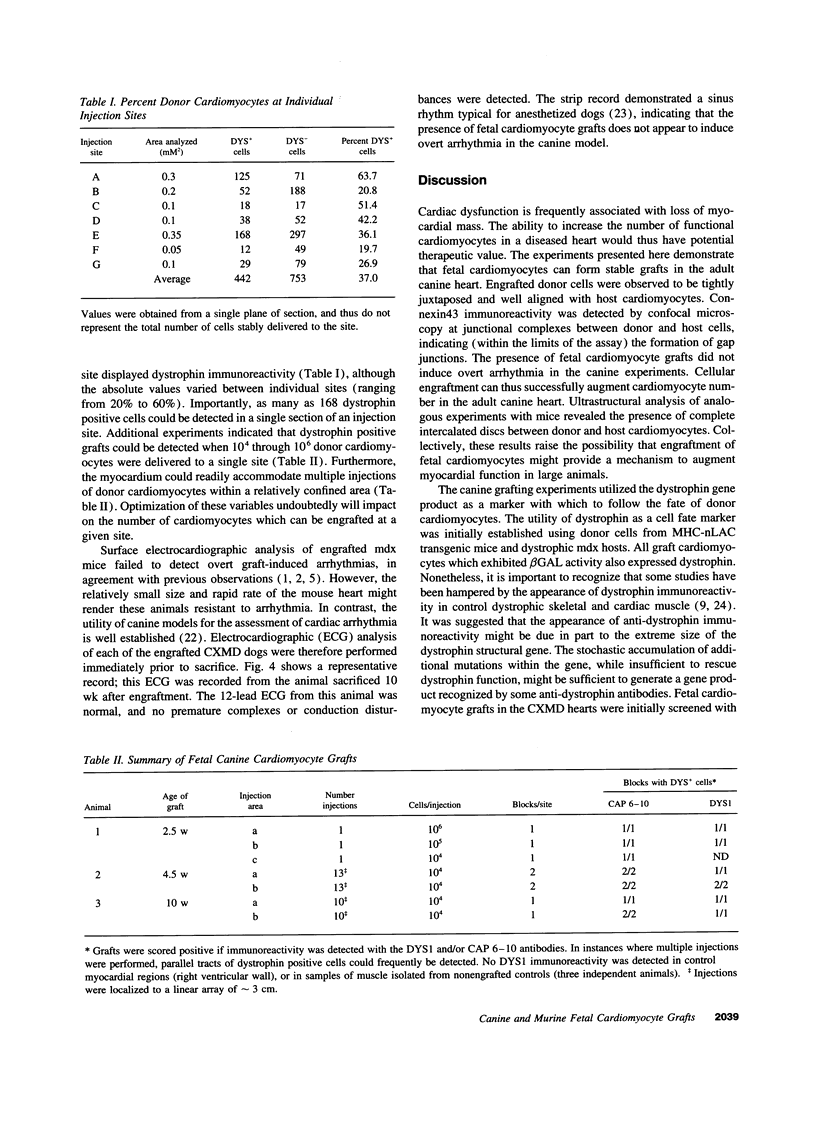
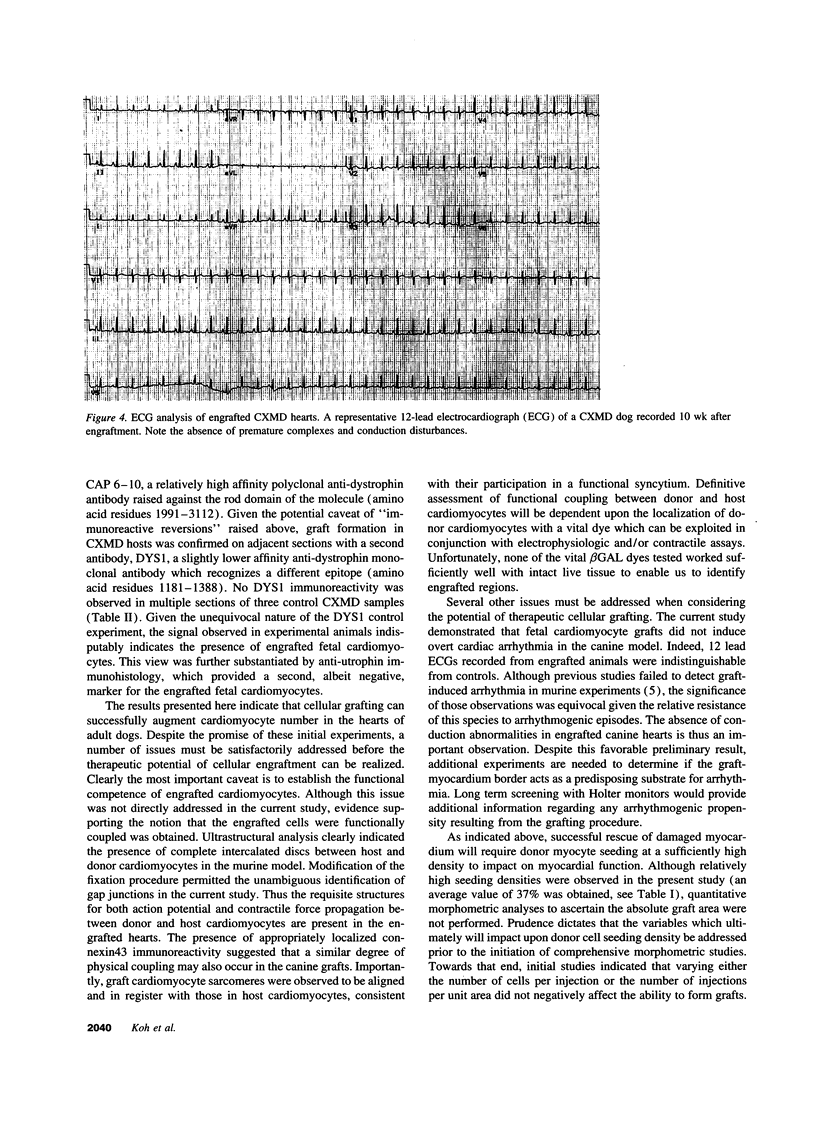
This report documents the formation of stable fetal cardiomyocyte grafts in the myocardium of dystrophic dogs. Preliminary experiments established that the dystrophin gene product could be used to follow the fate of engrafted cardiomyocytes in dystrophic mdx mice. Importantly, ultrastructural analyses revealed the presence of intercalated discs consisting of fascia adherens, desmosomes, and gap junctions at the donor-host cardiomyocyte border. To determine if isolated cardiomyocytes could form stable intracardiac grafts in a larger species, preparations of dissociated fetal canine cardiomyocytes were delivered into the hearts of adult CXMD (canine X-linked muscular dystrophy) dogs. CXMD dogs, like Duchenne muscular dystrophy patients and mdx mice, fail to express dystrophin in both cardiac and skeletal muscle. Engrafted fetal cardiomyocytes, identified by virtue of dystrophin immunoreactivity, were observed to be tightly juxtaposed with host cardiomyocytes as long as 10 wk after engraftment, the latest date analyzed. Confocal laser scanning microscopy revealed the presence of connexin43, a major constituent of the gap junction, at the donor-host cardiomyocyte border. The presence of intracardiac grafts was not associated with arrhythmogenesis in the CXMD model. These results indicate that fetal cardiomyocyte grafting can successfully augment cardiomyocyte number in larger animals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antzelevitch C., Sicouri S., Litovsky S. H., Lukas A., Krishnan S. C., Di Diego J. M., Gintant G. A., Liu D. W. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res. 1991 Dec;69(6):1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B. J., Winand N. J., Stedman H., Valentine B. A., Hoffman E. P., Kunkel L. M., Scott M. O., Fischbeck K. H., Kornegay J. N., Avery R. J. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988 Jul 14;334(6178):154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- Danko I., Chapman V., Wolff J. A. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992 Jul;32(1):128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991 Sep 20;66(6):1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Pavlath G. K., Lanctot A. M., Sharma K. R., Miller R. G., Steinman L., Blau H. M. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992 Apr 2;356(6368):435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Koh G. Y., Klug M. G., Soonpaa M. H., Field L. J. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993 Sep;92(3):1548–1554. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh G. Y., Soonpaa M. H., Klug M. G., Field L. J. Long-term survival of AT-1 cardiomyocyte grafts in syngeneic myocardium. Am J Physiol. 1993 May;264(5 Pt 2):H1727–H1733. doi: 10.1152/ajpheart.1993.264.5.H1727. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Campbell K. P. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994 Jan;17(1):2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Ervasti J. M., Ohlendieck K., Kahl S. D., Campbell K. P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992 Dec 10;360(6404):588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Nonaka I., Hirai S., Ozawa E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD-carriers and control subjects. J Neurol Sci. 1993 Oct;119(1):43–52. doi: 10.1016/0022-510x(93)90190-a. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Watt D. J., Sloper J. C., Partridge T. A. Partial correction of an inherited biochemical defect of skeletal muscle by grafts of normal muscle precursor cells. J Neurol Sci. 1988 Sep;86(2-3):137–147. doi: 10.1016/0022-510x(88)90093-7. [DOI] [PubMed] [Google Scholar]
- Olson E. N. Regulation of muscle transcription by the MyoD family. The heart of the matter. Circ Res. 1993 Jan;72(1):1–6. doi: 10.1161/01.res.72.1.1. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Sahashi K., Ibi T., Suoh H., Nakao N., Tashiro M., Marui K., Arahata K., Sugita H. Immunostaining of dystrophin and utrophin in skeletal muscle of dystrophinopathies. Intern Med. 1994 May;33(5):277–283. doi: 10.2169/internalmedicine.33.277. [DOI] [PubMed] [Google Scholar]
- Soonpaa M. H., Field L. J. Assessment of cardiomyocyte DNA synthesis during hypertrophy in adult mice. Am J Physiol. 1994 Apr;266(4 Pt 2):H1439–H1445. doi: 10.1152/ajpheart.1994.266.4.H1439. [DOI] [PubMed] [Google Scholar]
- Soonpaa M. H., Koh G. Y., Klug M. G., Field L. J. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994 Apr 1;264(5155):98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- Thompson L. Cell-transplant results under fire. Science. 1992 Jul 24;257(5069):472–474. doi: 10.1126/science.1636079. [DOI] [PubMed] [Google Scholar]
- Thompson L. Researchers call for time out on cell-transplant research. Science. 1992 Aug 7;257(5071):738–738. doi: 10.1126/science.1496389. [DOI] [PubMed] [Google Scholar]
- Valentine B. A., Cooper B. J., Cummings J. F., de Lahunta A. Canine X-linked muscular dystrophy: morphologic lesions. J Neurol Sci. 1990 Jun;97(1):1–23. doi: 10.1016/0022-510x(90)90095-5. [DOI] [PubMed] [Google Scholar]
- Valentine B. A., Cooper B. J., de Lahunta A., O'Quinn R., Blue J. T. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci. 1988 Dec;88(1-3):69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- Valentine B. A., Winand N. J., Pradhan D., Moise N. S., de Lahunta A., Kornegay J. N., Cooper B. J. Canine X-linked muscular dystrophy as an animal model of Duchenne muscular dystrophy: a review. Am J Med Genet. 1992 Feb 1;42(3):352–356. doi: 10.1002/ajmg.1320420320. [DOI] [PubMed] [Google Scholar]
- Watt D. J., Morgan J. E., Partridge T. A. Use of mononuclear precursor cells to insert allogeneic genes into growing mouse muscles. Muscle Nerve. 1984 Nov-Dec;7(9):741–750. doi: 10.1002/mus.880070908. [DOI] [PubMed] [Google Scholar]
- Zibaitis A., Greentree D., Ma F., Marelli D., Duong M., Chiu R. C. Myocardial regeneration with satellite cell implantation. Transplant Proc. 1994 Dec;26(6):3294–3294. [PubMed] [Google Scholar]
- Zipes D. P. Influence of myocardial ischemia and infarction on autonomic innervation of heart. Circulation. 1990 Oct;82(4):1095–1105. doi: 10.1161/01.cir.82.4.1095. [DOI] [PubMed] [Google Scholar]