Abstract
Fatal Plasmodium falciparum malaria is accompanied by systemic endothelial activation. To study endothelial activation directly during malaria and sepsis in vivo, the expression of cell adhesion molecules on dermal microvascular endothelium was examined in skin biopsies and correlated with plasma levels of soluble (circulating) ICAM-1, E-selectin, and VCAM-1 and the cytokine tumor necrosis factor (TNF)-alpha. Skin biopsies were obtained from 61 cases of severe malaria, 42 cases of uncomplicated malaria, 10 cases of severe systemic sepsis, and 17 uninfected controls. Systemic endothelial activation, represented by the up-regulation of inducible cell adhesion molecules (CAMs) on endothelium and increased levels of soluble CAMs (sCAMs), were seen in both severe and uncomplicated malaria and sepsis when compared with uninfected controls. Plasma levels of sICAM-1, sVCAM-1, and sE-selectin correlated positively with the severity of malaria whereas TNF-alpha was raised nonspecifically in malaria and sepsis. Immunohistochemical evidence of endothelial activation in skin biopsies did not correlate with sCAM levels or disease severity. This indicates a background of systemic endothelial activation, which occurs in both mild and severe malaria and sepsis. The levels of sCAMs in malaria are thus not an accurate reflection of endothelial cell expression of CAMs in a particular vascular bed, and other factors must influence their levels during disease.
Full text
PDF

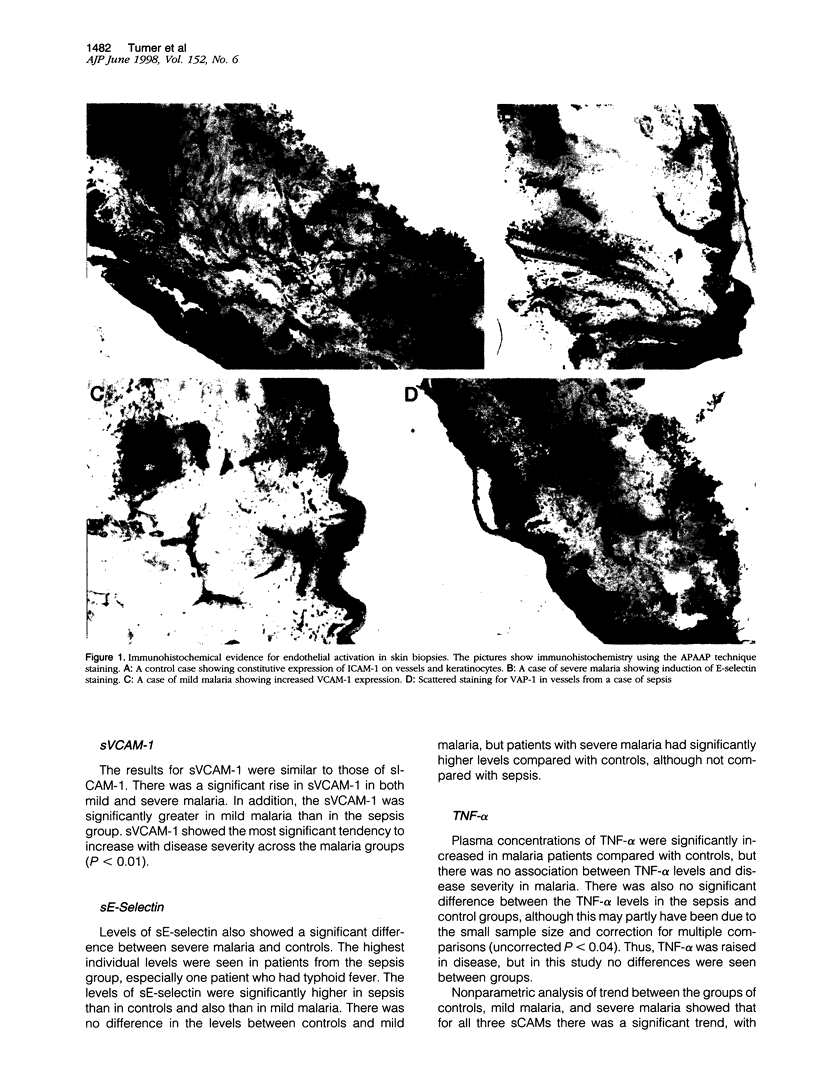
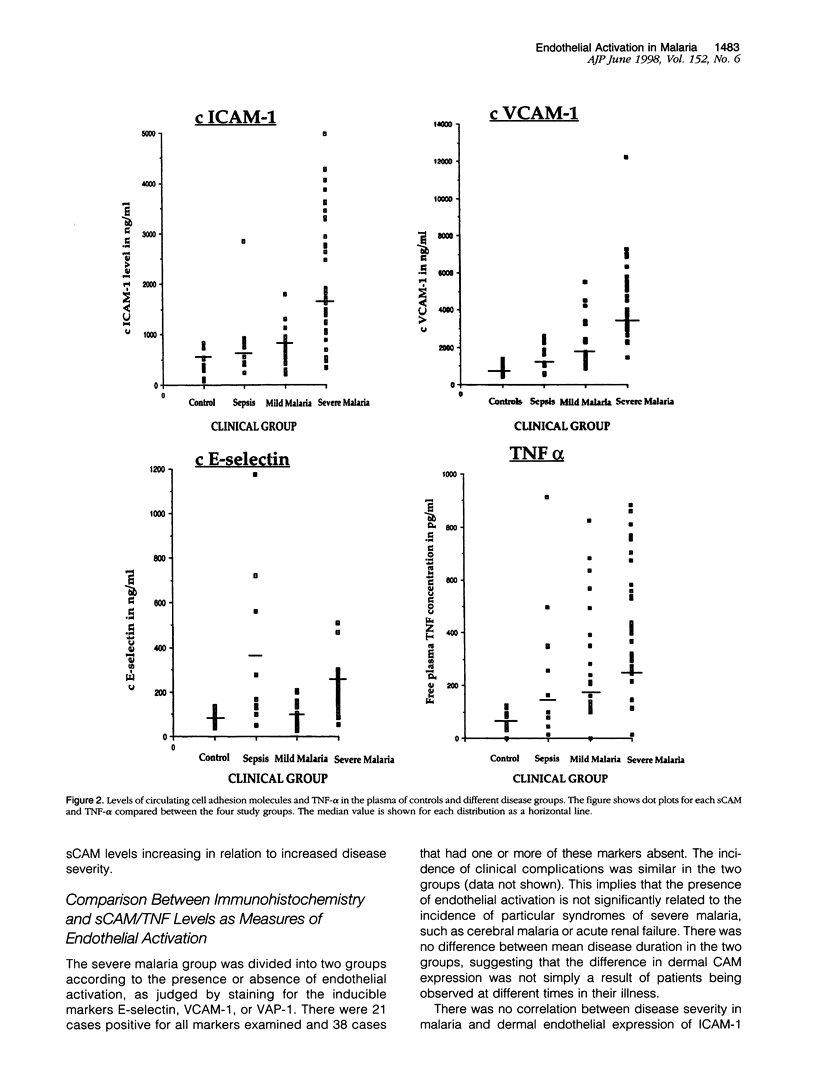


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Pongponratn E., Tegoshi T., Nakamura K., Nagatake T., Cochrane A., Ozaki L. S. A study on the pathogenesis of human cerebral malaria and cerebral babesiosis. Mem Inst Oswaldo Cruz. 1992;87 (Suppl 3):297–301. doi: 10.1590/s0074-02761992000700051. [DOI] [PubMed] [Google Scholar]
- Arvilommi A. M., Salmi M., Kalimo K., Jalkanen S. Lymphocyte binding to vascular endothelium in inflamed skin revisited: a central role for vascular adhesion protein-1 (VAP-1). Eur J Immunol. 1996 Apr;26(4):825–833. doi: 10.1002/eji.1830260415. [DOI] [PubMed] [Google Scholar]
- Berendt A. R. Sequestration and its discontents: infected erythrocyte-endothelial cell interactions in Plasmodium falciparum malaria. Res Immunol. 1993 Nov-Dec;144(9):740–762. doi: 10.1016/s0923-2494(93)80059-8. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Cowley H. C., Heney D., Gearing A. J., Hemingway I., Webster N. R. Increased circulating adhesion molecule concentrations in patients with the systemic inflammatory response syndrome: a prospective cohort study. Crit Care Med. 1994 Apr;22(4):651–657. doi: 10.1097/00003246-199404000-00022. [DOI] [PubMed] [Google Scholar]
- Craig A. G., Pinches R., Khan S., Roberts D. J., Turner G. D., Newbold C. I., Berendt A. R. Failure to block adhesion of Plasmodium falciparum-infected erythrocytes to ICAM-1 with soluble ICAM-1. Infect Immun. 1997 Nov;65(11):4580–4585. doi: 10.1128/iai.65.11.4580-4585.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloron P., Dumont N., Nyongabo T., Aubry P., Astagneau P., Ndarugirire F., Menetrier-Caux C., Burdin N., Brelivet J. C., Peyron F. Immunologic and biochemical alterations in severe falciparum malaria: relation to neurological symptoms and outcome. Clin Infect Dis. 1994 Sep;19(3):480–485. doi: 10.1093/clinids/19.3.480. [DOI] [PubMed] [Google Scholar]
- Donnelly S. C., Haslett C., Dransfield I., Robertson C. E., Carter D. C., Ross J. A., Grant I. S., Tedder T. F. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994 Jul 23;344(8917):215–219. doi: 10.1016/s0140-6736(94)92995-5. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Cheng J., Chang A., Taylor F. B., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993 May;142(5):1458–1470. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Reyes D., Craig A. G., Kyes S. A., Peshu N., Snow R. W., Berendt A. R., Marsh K., Newbold C. I. A high frequency African coding polymorphism in the N-terminal domain of ICAM-1 predisposing to cerebral malaria in Kenya. Hum Mol Genet. 1997 Aug;6(8):1357–1360. doi: 10.1093/hmg/6.8.1357. [DOI] [PubMed] [Google Scholar]
- Fries J. W., Williams A. J., Atkins R. C., Newman W., Lipscomb M. F., Collins T. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am J Pathol. 1993 Sep;143(3):725–737. [PMC free article] [PubMed] [Google Scholar]
- Gearing A. J., Hemingway I., Pigott R., Hughes J., Rees A. J., Cashman S. J. Soluble forms of vascular adhesion molecules, E-selectin, ICAM-1, and VCAM-1: pathological significance. Ann N Y Acad Sci. 1992 Dec 4;667:324–331. doi: 10.1111/j.1749-6632.1992.tb51633.x. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993 Oct;14(10):506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- Graninger W., Prada J., Neifer S., Zotter G., Thalhammer F., Kremsner P. G. Upregulation of ICAM-I by Plasmodium falciparum: in vitro and in vivo studies. J Clin Pathol. 1994 Jul;47(7):653–656. doi: 10.1136/jcp.47.7.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Groves R. W., Allen M. H., Ross E. L., Barker J. N., MacDonald D. M. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol. 1995 Mar;132(3):345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Herskowitz A., Mayne A. E., Willoughby S. B., Kanter K., Ansari A. A. Patterns of myocardial cell adhesion molecule expression in human endomyocardial biopsies after cardiac transplantation. Induced ICAM-1 and VCAM-1 related to implantation and rejection. Am J Pathol. 1994 Nov;145(5):1082–1094. [PMC free article] [PubMed] [Google Scholar]
- Hviid L., Theander T. G., Elhassan I. M., Jensen J. B. Increased plasma levels of soluble ICAM-1 and ELAM-1 (E-selectin) during acute Plasmodium falciparum malaria. Immunol Lett. 1993 Apr;36(1):51–58. doi: 10.1016/0165-2478(93)90068-d. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Morris-Jones S., Rønn A., Hviid L., Theander T. G., Elhassan I. M., Bygbjerg I. C., Greenwood B. M. Increased plasma concentrations of sICAM-1, sVCAM-1 and sELAM-1 in patients with Plasmodium falciparum or P. vivax malaria and association with disease severity. Immunology. 1994 Dec;83(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- McGuire W., Hill A. V., Greenwood B. M., Kwiatkowski D. Circulating ICAM-1 levels in falciparum malaria are high but unrelated to disease severity. Trans R Soc Trop Med Hyg. 1996 May-Jun;90(3):274–276. doi: 10.1016/s0035-9203(96)90244-8. [DOI] [PubMed] [Google Scholar]
- Norton J., Sloane J. P., Delia D., Greaves M. F. Reciprocal expression of CD34 and cell adhesion molecule ELAM-1 on vascular endothelium in acute cutaneous graft-versus-host disease. J Pathol. 1993 Jun;170(2):173–177. doi: 10.1002/path.1711700213. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Tegoshi T., Maeno Y., Benjamin C., Ho M., Kan K. E., Thway Y., Win K., Aikawa M., Lobb R. R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992 Oct 1;176(4):1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasloske B. L., Howard R. J. Malaria, the red cell, and the endothelium. Annu Rev Med. 1994;45:283–295. doi: 10.1146/annurev.med.45.1.283. [DOI] [PubMed] [Google Scholar]
- Petzelbauer P., Bender J. R., Wilson J., Pober J. S. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993 Nov 1;151(9):5062–5072. [PubMed] [Google Scholar]
- Pigott R., Dillon L. P., Hemingway I. H., Gearing A. J. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun. 1992 Sep 16;187(2):584–589. doi: 10.1016/0006-291x(92)91234-h. [DOI] [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Pongponratn E., Riganti M., Punpoowong B., Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991 Feb;44(2):168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Swerlick R. A., Lee K. H., Wick T. M., Lawley T. J. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol. 1992 Jan 1;148(1):78–83. [PubMed] [Google Scholar]
- Tanio J. W., Basu C. B., Albelda S. M., Eisen H. J. Differential expression of the cell adhesion molecules ICAM-1, VCAM-1, and E-selectin in normal and posttransplantation myocardium. Cell adhesion molecule expression in human cardiac allografts. Circulation. 1994 Apr;89(4):1760–1768. doi: 10.1161/01.cir.89.4.1760. [DOI] [PubMed] [Google Scholar]
- Turner G. D., Morrison H., Jones M., Davis T. M., Looareesuwan S., Buley I. D., Gatter K. C., Newbold C. I., Pukritayakamee S., Nagachinta B. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994 Nov;145(5):1057–1069. [PMC free article] [PubMed] [Google Scholar]
- Uccini S., Ruco L. P., Monardo F., La Parola I. L., Cerimele D., Baroni C. D. Molecular mechanisms involved in intraepithelial lymphocyte migration: a comparative study in skin and tonsil. J Pathol. 1993 Apr;169(4):413–419. doi: 10.1002/path.1711690405. [DOI] [PubMed] [Google Scholar]
- White N. J., Ho M. The pathophysiology of malaria. Adv Parasitol. 1992;31:83–173. doi: 10.1016/s0065-308x(08)60021-4. [DOI] [PubMed] [Google Scholar]