Abstract
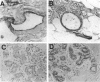
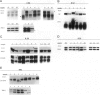
Breast cancer is believed to develop as multiple genetic abnormalities accumulate, each conferring some growth advantage, but the timing and nature of the earliest steps in this progression are not yet elucidated. Proliferative breast lesions, associated with an increased risk of breast cancer although considered benign, recently were shown to contain clonal genetic abnormalities. Therefore, we hypothesized that clonal genetic abnormalities might be detectable before any phenotypic abnormalities are evident, ie, in histologically normal breast tissue. We examined DNA extracted from 95 normal-appearing breast ducts or terminal ductal-lobular units from 20 individuals at varying degrees of risk (those undergoing reduction mammoplasties, those with atypical hyperplastic proliferative lesions, and those already diagnosed with breast cancer). Using nine microsatellite markers, we sought evidence of genetic instability or of allelic imbalance (most likely representing loss of heterozygosity). We found genetically abnormal clones in 21/95 (22%) seemingly normal samples from 10/20 (50%) women from all three risk groups. In women under age 50, trends toward increased rates of abnormalities were noted with increased cancer risk. The abnormalities identified were more likely to be at sites of known or postulated tumor suppressor genes rather than at random or neutral loci. Our data indicate that genetic abnormalities potentially critical to breast tumorigenesis accumulate before pathological detection even of high-risk lesions and are detectable in tissue that is not only histologically benign but also completely normal.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldaz C. M., Chen T., Sahin A., Cunningham J., Bondy M. Comparative allelotype of in situ and invasive human breast cancer: high frequency of microsatellite instability in lobular breast carcinomas. Cancer Res. 1995 Sep 15;55(18):3976–3981. [PubMed] [Google Scholar]
- Ali I. U., Lidereau R., Theillet C., Callahan R. Reduction to homozygosity of genes on chromosome 11 in human breast neoplasia. Science. 1987 Oct 9;238(4824):185–188. doi: 10.1126/science.3659909. [DOI] [PubMed] [Google Scholar]
- Brentnall T. A., Crispin D. A., Bronner M. P., Cherian S. P., Hueffed M., Rabinovitch P. S., Rubin C. E., Haggitt R. C., Boland C. R. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996 Mar 15;56(6):1237–1240. [PubMed] [Google Scholar]
- Champème M. H., Bièche I., Beuzelin M., Lidereau R. Loss of heterozygosity on 7q31 occurs early during breast tumorigenesis. Genes Chromosomes Cancer. 1995 Apr;12(4):304–306. doi: 10.1002/gcc.2870120411. [DOI] [PubMed] [Google Scholar]
- Chen T., Sahin A., Aldaz C. M. Deletion map of chromosome 16q in ductal carcinoma in situ of the breast: refining a putative tumor suppressor gene region. Cancer Res. 1996 Dec 15;56(24):5605–5609. [PubMed] [Google Scholar]
- Chuaqui R. F., Zhuang Z., Emmert-Buck M. R., Liotta L. A., Merino M. J. Analysis of loss of heterozygosity on chromosome 11q13 in atypical ductal hyperplasia and in situ carcinoma of the breast. Am J Pathol. 1997 Jan;150(1):297–303. [PMC free article] [PubMed] [Google Scholar]
- Contegiacomo A., Palmirotta R., De Marchis L., Pizzi C., Mastranzo P., Delrio P., Petrella G., Figliolini M., Bianco A. R., Frati L. Microsatellite instability and pathological aspects of breast cancer. Int J Cancer. 1995 Aug 22;64(4):264–268. doi: 10.1002/ijc.2910640409. [DOI] [PubMed] [Google Scholar]
- Dawson P. J., Baekey P. A., Clark R. A. Mechanisms of multifocal breast cancer: an immunocytochemical study. Hum Pathol. 1995 Sep;26(9):965–969. doi: 10.1016/0046-8177(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Deng G., Lu Y., Zlotnikov G., Thor A. D., Smith H. S. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996 Dec 20;274(5295):2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Devilee P., Cornelisse C. J. Somatic genetic changes in human breast cancer. Biochim Biophys Acta. 1994 Dec 30;1198(2-3):113–130. doi: 10.1016/0304-419x(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Eyfjörd J. E., Thorlacius S., Steinarsdottir M., Valgardsdottir R., Ogmundsdottir H. M., Anamthawat-Jonsson K. p53 abnormalities and genomic instability in primary human breast carcinomas. Cancer Res. 1995 Feb 1;55(3):646–651. [PubMed] [Google Scholar]
- Fujii H., Marsh C., Cairns P., Sidransky D., Gabrielson E. Genetic divergence in the clonal evolution of breast cancer. Cancer Res. 1996 Apr 1;56(7):1493–1497. [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Hackman P., Gabbani G., Osterholm A. M., Hellgren D., Lambert B. Spontaneous length variation in microsatellite DNA from human T-cell clones. Genes Chromosomes Cancer. 1995 Nov;14(3):215–219. doi: 10.1002/gcc.2870140310. [DOI] [PubMed] [Google Scholar]
- Jonason A. S., Kunala S., Price G. J., Restifo R. J., Spinelli H. M., Persing J. A., Leffell D. J., Tarone R. E., Brash D. E. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):14025–14029. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson M., Johannsson O., Borg A. Infrequent occurrence of microsatellite instability in sporadic and familial breast cancer. Eur J Cancer. 1995 Dec;31A(13-14):2330–2334. doi: 10.1016/0959-8049(95)00447-5. [DOI] [PubMed] [Google Scholar]
- Kasami M., Vnencak-Jones C. L., Manning S., Dupont W. D., Page D. L. Loss of heterozygosity and microsatellite instability in breast hyperplasia. No obligate correlation of these genetic alterations with subsequent malignancy. Am J Pathol. 1997 Jun;150(6):1925–1932. [PMC free article] [PubMed] [Google Scholar]
- Kerangueven F., Eisinger F., Noguchi T., Allione F., Wargniez V., Eng C., Padberg G., Theillet C., Jacquemier J., Longy M. Loss of heterozygosity in human breast carcinomas in the ataxia telangiectasia, Cowden disease and BRCA1 gene regions. Oncogene. 1997 Jan 23;14(3):339–347. doi: 10.1038/sj.onc.1200818. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996 Oct 18;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Lakhani S. R., Collins N., Stratton M. R., Sloane J. P. Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol. 1995 Jul;48(7):611–615. doi: 10.1136/jcp.48.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani S. R., Slack D. N., Hamoudi R. A., Collins N., Stratton M. R., Sloane J. P. Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab Invest. 1996 Jan;74(1):129–135. [PubMed] [Google Scholar]
- Li L., Li X., Francke U., Cohen S. N. The TSG101 tumor susceptibility gene is located in chromosome 11 band p15 and is mutated in human breast cancer. Cell. 1997 Jan 10;88(1):143–154. doi: 10.1016/s0092-8674(00)81866-8. [DOI] [PubMed] [Google Scholar]
- Mashal R. D., Lester S. C., Sklar J. Clonal analysis by study of X chromosome inactivation in formalin-fixed paraffin-embedded tissue. Cancer Res. 1993 Oct 1;53(19):4676–4679. [PubMed] [Google Scholar]
- Munn K. E., Walker R. A., Menasce L., Varley J. M. Allelic imbalance in the region of the BRCA1 gene in ductal carcinoma in situ of the breast. Br J Cancer. 1996 Mar;73(5):636–639. doi: 10.1038/bjc.1996.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter G. L., Boynton K. A. PCR bias in amplification of androgen receptor alleles, a trinucleotide repeat marker used in clonality studies. Nucleic Acids Res. 1995 Apr 25;23(8):1411–1418. doi: 10.1093/nar/23.8.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S., Motomura K., Inaji H., Imaoka S., Koyama H. Clonal analysis of predominantly intraductal carcinoma and precancerous lesions of the breast by means of polymerase chain reaction. Cancer Res. 1994 Apr 1;54(7):1849–1853. [PubMed] [Google Scholar]
- Ozbun M. A., Butel J. S. Tumor suppressor p53 mutations and breast cancer: a critical analysis. Adv Cancer Res. 1995;66:71–141. doi: 10.1016/s0065-230x(08)60252-3. [DOI] [PubMed] [Google Scholar]
- Pandis N., Jin Y., Gorunova L., Petersson C., Bardi G., Idvall I., Johansson B., Ingvar C., Mandahl N., Mitelman F. Chromosome analysis of 97 primary breast carcinomas: identification of eight karyotypic subgroups. Genes Chromosomes Cancer. 1995 Mar;12(3):173–185. doi: 10.1002/gcc.2870120304. [DOI] [PubMed] [Google Scholar]
- Patel U., Grundfest-Broniatowski S., Gupta M., Banerjee S. Microsatellite instabilities at five chromosomes in primary breast tumors. Oncogene. 1994 Dec;9(12):3695–3700. [PubMed] [Google Scholar]
- Petersson C., Pandis N., Mertens F., Adeyinka A., Ingvar C., Ringberg A., Idvall I., Bondeson L., Borg A., Olsson H. Chromosome aberrations in prophylactic mastectomies from women belonging to breast cancer families. Genes Chromosomes Cancer. 1996 Jul;16(3):185–188. doi: 10.1002/(SICI)1098-2264(199607)16:3<185::AID-GCC5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ren Z. P., Hedrum A., Pontén F., Nistér M., Ahmadian A., Lundeberg J., Uhlén M., Pontén J. Human epidermal cancer and accompanying precursors have identical p53 mutations different from p53 mutations in adjacent areas of clonally expanded non-neoplastic keratinocytes. Oncogene. 1996 Feb 15;12(4):765–773. [PubMed] [Google Scholar]
- Rosenberg C. L., Larson P. S., Romo J. D., De Las Morenas A., Faller D. V. Microsatellite alterations indicating monoclonality in atypical hyperplasias associated with breast cancer. Hum Pathol. 1997 Feb;28(2):214–219. doi: 10.1016/s0046-8177(97)90109-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg C. L., de las Morenas A., Huang K., Cupples L. A., Faller D. V., Larson P. S. Detection of monoclonal microsatellite alterations in atypical breast hyperplasia. J Clin Invest. 1996 Sep 1;98(5):1095–1100. doi: 10.1172/JCI118890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M. R., Pandis N., Bardi G., Andersen J. A., Heim S. Karyotypic comparisons of multiple tumorous and macroscopically normal surrounding tissue samples from patients with breast cancer. Cancer Res. 1996 Feb 15;56(4):855–859. [PubMed] [Google Scholar]
- Teixeira M. R., Pandis N., Bardi G., Andersen J. A., Mitelman F., Heim S. Clonal heterogeneity in breast cancer: karyotypic comparisons of multiple intra- and extra-tumorous samples from 3 patients. Int J Cancer. 1995 Sep 27;63(1):63–68. doi: 10.1002/ijc.2910630113. [DOI] [PubMed] [Google Scholar]
- Toyama T., Iwase H., Yamashita H., Iwata H., Yamashita T., Ito K., Hara Y., Suchi M., Kato T., Nakamura T. Microsatellite instability in sporadic human breast cancers. Int J Cancer. 1996 Nov 15;68(4):447–451. doi: 10.1002/(SICI)1097-0215(19961115)68:4<447::AID-IJC8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Tsai Y. C., Lu Y., Nichols P. W., Zlotnikov G., Jones P. A., Smith H. S. Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res. 1996 Jan 15;56(2):402–404. [PubMed] [Google Scholar]
- Waridel F., Estreicher A., Bron L., Flaman J. M., Fontolliet C., Monnier P., Frebourg T., Iggo R. Field cancerisation and polyclonal p53 mutation in the upper aero-digestive tract. Oncogene. 1997 Jan 16;14(2):163–169. doi: 10.1038/sj.onc.1200812. [DOI] [PubMed] [Google Scholar]
- Wooster R., Cleton-Jansen A. M., Collins N., Mangion J., Cornelis R. S., Cooper C. S., Gusterson B. A., Ponder B. A., von Deimling A., Wiestler O. D. Instability of short tandem repeats (microsatellites) in human cancers. Nat Genet. 1994 Feb;6(2):152–156. doi: 10.1038/ng0294-152. [DOI] [PubMed] [Google Scholar]
- Yee C. J., Roodi N., Verrier C. S., Parl F. F. Microsatellite instability and loss of heterozygosity in breast cancer. Cancer Res. 1994 Apr 1;54(7):1641–1644. [PubMed] [Google Scholar]