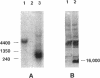
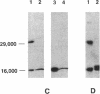
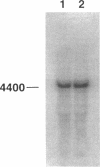
Abstract
To determine whether the product of the recently cloned ob gene functions as an adipose-related satiety factor, recombinant murine ob protein was administered intraperitoneally to ob/ob mice. Monomeric ob protein given as single morning injections to groups of three animals at seven doses ranging from 5 to 100 micrograms reduced 24-h chow consumption in a dose-dependent manner from values of 81 +/- 6.8% of control (10-micrograms dose, P = 0.04) to 29 +/- 7.7% of control (100-micrograms dose, P < 0.0001). Daily injections of 80 micrograms of ob protein into six ob/ob mice for 2 wk led to an 11 +/- 1.6% decrease in body weight (P = 0.0009) and suppressed feeding to 26 +/- 4.9% of baseline (P < 0.0001), with significant reduction of serum insulin and glucose levels. The effect of recombinant ob protein on feeding was not augmented by cofactors secreted by adipose tissue, nor did exposure of adipose tissue to ob protein affect intracellular ob mRNA levels. Posttranslational modification of ob protein was not required for activity; however, addition of a hexahistidine tag to the amino terminus of the mature ob protein resulted in prolonged suppression of feeding after injection into ob/ob mice. These results demonstrate a direct effect of the ob protein to suppress feeding in the ob/ob mouse and suggest that this molecule plays a critical role in regulating total body fat content.
Full text
PDF

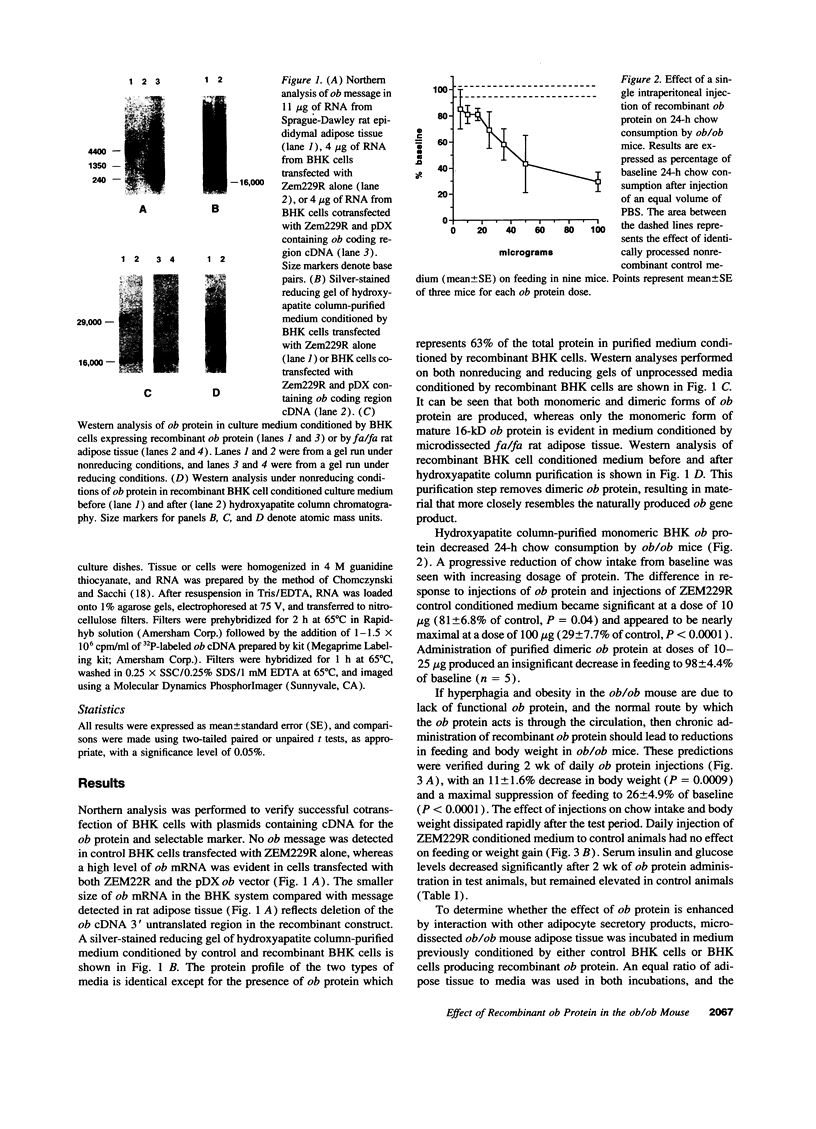


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray G. A., York D. A. Hypothalamic and genetic obesity in experimental animals: an autonomic and endocrine hypothesis. Physiol Rev. 1979 Jul;59(3):719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973 Aug;9(4):294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969 Nov;217(5):1298–1304. doi: 10.1152/ajplegacy.1969.217.5.1298. [DOI] [PubMed] [Google Scholar]
- Considine R. V., Considine E. L., Williams C. J., Nyce M. R., Magosin S. A., Bauer T. L., Rosato E. L., Colberg J., Caro J. F. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995 Jun;95(6):2986–2988. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. C., Rudinski M. S., Schach B. G., Berkner K. L., Kumar A. A., Hagen F. S., Sprecher C. A., Insley M. Y., Davie E. W. Propeptide of human protein C is necessary for gamma-carboxylation. Biochemistry. 1987 Nov 3;26(22):7003–7011. doi: 10.1021/bi00396a022. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- HERVEY G. R. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959 Mar 3;145(2):336–352. doi: 10.1113/jphysiol.1959.sp006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. B., Kasser T. R., Martin R. J. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. J Nutr. 1986 Dec;116(12):2536–2546. doi: 10.1093/jn/116.12.2536. [DOI] [PubMed] [Google Scholar]
- Harris R. B., Martin R. J. Recovery of body weight from below "set point" in mature female rats. J Nutr. 1984 Jun;114(6):1143–1150. doi: 10.1093/jn/114.6.1143. [DOI] [PubMed] [Google Scholar]
- Hubert H. B., Feinleib M., McNamara P. M., Castelli W. P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983 May;67(5):968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- Hulsey M. G., Martin R. J. An anorectic agent from adipose tissue of overfed rats: effects on feeding behavior. Physiol Behav. 1992 Dec;52(6):1141–1149. doi: 10.1016/0031-9384(92)90473-f. [DOI] [PubMed] [Google Scholar]
- KENNEDY G. C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953 Jan 15;140(901):578–596. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- Kalra S. P., Dube M. G., Sahu A., Phelps C. P., Kalra P. S. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10931–10935. doi: 10.1073/pnas.88.23.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesey R. E., Powley T. L. The regulation of body weight. Annu Rev Psychol. 1986;37:109–133. doi: 10.1146/annurev.ps.37.020186.000545. [DOI] [PubMed] [Google Scholar]
- Lindsted K., Tonstad S., Kuzma J. W. Body mass index and patterns of mortality among Seventh-day Adventist men. Int J Obes. 1991 Jun;15(6):397–406. [PubMed] [Google Scholar]
- Mrosovsky N. Body fat: what is regulated? Physiol Behav. 1986;38(3):407–414. doi: 10.1016/0031-9384(86)90113-7. [DOI] [PubMed] [Google Scholar]
- Murakami T., Shima K. Cloning of rat obese cDNA and its expression in obese rats. Biochem Biophys Res Commun. 1995 Apr 26;209(3):944–952. doi: 10.1006/bbrc.1995.1589. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Woods S. C. Regulation of food intake and body weight in insulin. Diabetologia. 1981 Mar;20 (Suppl):274–280. [PubMed] [Google Scholar]
- Rink T. J. Genetics. In search of a satiety factor. Nature. 1994 Dec 1;372(6505):406–407. doi: 10.1038/372406a0. [DOI] [PubMed] [Google Scholar]
- Stanley B. G., Kyrkouli S. E., Lampert S., Leibowitz S. F. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986 Nov-Dec;7(6):1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Weigle D. S. Appetite and the regulation of body composition. FASEB J. 1994 Mar 1;8(3):302–310. doi: 10.1096/fasebj.8.3.8143936. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]