Abstract
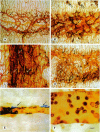
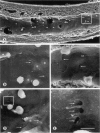
Exposure of sensitized individuals to antigen can induce allergic responses in the respiratory tract, manifested by early and late phases of vasodilatation, plasma leakage, leukocyte influx, and bronchoconstriction. Similar responses can occur in the skin, eye, and gastrointestinal tract. The early-phase response involves mast cell mediators and the late-phase response is leukocyte dependent, but the mechanism of leakage is not understood. We sought to identify the leaky blood vessels, to determine whether these vessels contained endothelial gaps, and to analyze the relationship of the gaps to adherent leukocytes, using biotinylated lectins or silver nitrate to stain the cells in situ and Monastral blue as a tracer to quantify plasma leakage. Most of the leakage occurred in postcapillary venules (< 40-microns diameter), whereas most of the leukocyte migration (predominantly neutrophils) occurred in collecting venules. Capillaries and arterioles did not leak. Endothelial gaps were found in the leaky venules, both by silver nitrate staining and by scanning electron microscopy, and 94% of the gaps were distinct from sites of leukocyte adhesion or migration. We conclude that endothelial gaps contribute to both early and late phases of plasma leakage induced by antigen, but most leakage occurs upstream to sites of leukocyte adhesion.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluk P., Hirata A., Thurston G., Fujiwara T., Neal C. R., Michel C. C., McDonald D. M. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am J Physiol. 1997 Jan;272(1 Pt 1):L155–L170. doi: 10.1152/ajplung.1997.272.1.L155. [DOI] [PubMed] [Google Scholar]
- Benhamou M., Berenstein E. H., Jouvin M. H., Siraganian R. P. The receptor with high affinity for IgE on rat mast cells is a functional receptor for rat IgG2a. Mol Immunol. 1994 Oct;31(14):1089–1097. doi: 10.1016/0161-5890(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Bolton P. B., Lefevre P., McDonald D. M. Salmeterol reduces early- and late-phase plasma leakage and leukocyte adhesion in rat airways. Am J Respir Crit Care Med. 1997 Apr;155(4):1428–1435. doi: 10.1164/ajrccm.155.4.9105089. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Zerhouni E. A., Mitzner W. Airway edema potentiates airway reactivity. J Appl Physiol (1985) 1995 Oct;79(4):1242–1248. doi: 10.1152/jappl.1995.79.4.1242. [DOI] [PubMed] [Google Scholar]
- Chiba Y., Misawa M. Strain differences in change in airway responsiveness after repeated antigenic challenge in three strains of rats. Gen Pharmacol. 1993 Sep;24(5):1265–1272. doi: 10.1016/0306-3623(93)90379-c. [DOI] [PubMed] [Google Scholar]
- Cuénoud H. F., Joris I., Langer R. S., Majno G. Focal arteriolar insudation. A response of arterioles to chronic nonspecific irritation. Am J Pathol. 1987 Jun;127(3):592–604. [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Kohn S., Morgan E. S., Fox P., Nagy J. A., Dvorak H. F. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol. 1996 Jan;59(1):100–115. [PubMed] [Google Scholar]
- Elwood W., Barnes P. J., Chung K. F. Airway hyperresponsiveness is associated with inflammatory cell infiltration in allergic brown-Norway rats. Int Arch Allergy Immunol. 1992;99(1):91–97. doi: 10.1159/000236340. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Luts A., Persson C. G. Appearance of airway absorption and exudation tracers in guinea pig tracheobronchial lymph nodes. J Appl Physiol (1985) 1993 Feb;74(2):817–824. doi: 10.1152/jappl.1993.74.2.817. [DOI] [PubMed] [Google Scholar]
- Galli S. J. Complexity and redundancy in the pathogenesis of asthma: reassessing the roles of mast cells and T cells. J Exp Med. 1997 Aug 4;186(3):343–347. doi: 10.1084/jem.186.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLEY J. V. ACUTE INFLAMMATION: THE EFFECT OF CONCURRENT LEUCOCYTIC EMIGRATION AND INCREASED PERMEABILITY ON PARTICLE RETENTION BY THE VASCULAR WALL. Br J Exp Pathol. 1964 Dec;45:627–633. [PMC free article] [PubMed] [Google Scholar]
- Hirata A., Baluk P., Fujiwara T., McDonald D. M. Location of focal silver staining at endothelial gaps in inflamed venules examined by scanning electron microscopy. Am J Physiol. 1995 Sep;269(3 Pt 1):L403–L418. doi: 10.1152/ajplung.1995.269.3.L403. [DOI] [PubMed] [Google Scholar]
- Joris I., Cuénoud H. F., Doern G. V., Underwood J. M., Majno G. Capillary leakage in inflammation. A study by vascular labeling. Am J Pathol. 1990 Dec;137(6):1353–1363. [PMC free article] [PubMed] [Google Scholar]
- Lewis R. E., Granger H. J. Diapedesis and the permeability of venous microvessels to protein macromolecules: the impact of leukotriene B4 (LTB4). Microvasc Res. 1988 Jan;35(1):27–47. doi: 10.1016/0026-2862(88)90048-9. [DOI] [PubMed] [Google Scholar]
- Lima M. C., Prouvost-Danon A., e Silva P. M., Chagas M. S., Calheiros A. S., Cordeiro R. S., Latine D., Bazin H., Ryan U. S., Martins M. A. Studies on the mechanisms involved in antigen-evoked pleural inflammation in rats: contribution of IgE and complement. J Leukoc Biol. 1997 Mar;61(3):286–292. doi: 10.1002/jlb.61.3.286. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G. Maude Abbott Lecture--1991. The capillary then and now: an overview of capillary pathology. Mod Pathol. 1992 Jan;5(1):9–22. [PubMed] [Google Scholar]
- McDonald D. M. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol. 1994 Jan;266(1 Pt 1):L61–L83. doi: 10.1152/ajplung.1994.266.1.L61. [DOI] [PubMed] [Google Scholar]
- Mekori Y. A., Galli S. J. [125I]fibrin deposition occurs at both early and late intervals of IgE-dependent or contact sensitivity reactions elicited in mouse skin. Mast cell-dependent augmentation of fibrin deposition at early intervals in combined IgE-dependent and contact sensitivity reactions. J Immunol. 1990 Dec 1;145(11):3719–3727. [PubMed] [Google Scholar]
- Mu H. H., Sewell W. A. Enhancement of interleukin-4 production by pertussis toxin. Infect Immun. 1993 Jul;61(7):2834–2840. doi: 10.1128/iai.61.7.2834-2840.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal C. R., Michel C. C. Transcellular gaps in microvascular walls of frog and rat when permeability is increased by perfusion with the ionophore A23187. J Physiol. 1995 Oct 15;488(Pt 2):427–437. doi: 10.1113/jphysiol.1995.sp020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne P. M., Dolovich J., Hargreave F. E. Late asthmatic responses. Am Rev Respir Dis. 1987 Sep;136(3):740–751. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Bazin H., Platteau B., van der Straeten M. The influence of antigen dose on IgE production in different rat strains. Immunology. 1979 Jan;36(1):151–157. [PMC free article] [PubMed] [Google Scholar]
- Peers S. H., Duncan G. S., Flower R. J. Development of specific antibody and in vivo response to antigen in different rat strains: effect of dexamethasone and importance of endogenous corticosteroids. Agents Actions. 1993 Jul;39(3-4):174–181. doi: 10.1007/BF01998971. [DOI] [PubMed] [Google Scholar]
- Persson C. G. Plasma exudation in the airways: mechanisms and function. Eur Respir J. 1991 Nov;4(10):1268–1274. [PubMed] [Google Scholar]
- Piechuta H., Smith M. E., Share N. N., Holme G. The respiratory response of sensitized rats to challenge with antigen aerosols. Immunology. 1979 Oct;38(2):385–392. [PMC free article] [PubMed] [Google Scholar]
- Pietra G. G., Johns L. W. Confocal- and electron-microscopic localization of FITC-albumin in H2O2-induced pulmonary edema. J Appl Physiol (1985) 1996 Jan;80(1):182–190. doi: 10.1152/jappl.1996.80.1.182. [DOI] [PubMed] [Google Scholar]
- Renzi P. M., Olivenstein R., Martin J. G. Inflammatory cell populations in the airways and parenchyma after antigen challenge in the rat. Am Rev Respir Dis. 1993 Apr;147(4):967–974. doi: 10.1164/ajrccm/147.4.967. [DOI] [PubMed] [Google Scholar]
- Sapienza S., Eidelman D. H., Renzi P. M., Martin J. G. Role of leukotriene D4 in the early and late pulmonary responses of rats to allergen challenge. Am Rev Respir Dis. 1990 Aug;142(2):353–358. doi: 10.1164/ajrccm/142.2.353. [DOI] [PubMed] [Google Scholar]
- Sekiya S., Yamashita T., Sendo F. Suppression of late phase enhanced vascular permeability in rats by selective depletion of neutrophils with a monoclonal antibody. J Leukoc Biol. 1990 Sep;48(3):258–265. doi: 10.1002/jlb.48.3.258. [DOI] [PubMed] [Google Scholar]
- Simionescu N. Cellular aspects of transcapillary exchange. Physiol Rev. 1983 Oct;63(4):1536–1579. doi: 10.1152/physrev.1983.63.4.1536. [DOI] [PubMed] [Google Scholar]
- Smith S. R., Petillo J. IgE production in five inbred rat strains following immunization with alum-precipitated egg albumin. Int Arch Allergy Appl Immunol. 1976;52(1-4):21–31. doi: 10.1159/000231663. [DOI] [PubMed] [Google Scholar]
- Tam E. K., Calonico L. D., Nadel J. A., McDonald D. M. Globule leukocytes and mast cells in the rat trachea: their number, distribution, and response to compound 48/80 and dexamethasone. Anat Embryol (Berl) 1988;178(2):107–118. doi: 10.1007/BF02463644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G., Baluk P., Hirata A., McDonald D. M. Permeability-related changes revealed at endothelial cell borders in inflamed venules by lectin binding. Am J Physiol. 1996 Dec;271(6 Pt 2):H2547–H2562. doi: 10.1152/ajpheart.1996.271.6.H2547. [DOI] [PubMed] [Google Scholar]
- Turner D. J., Myron P., Powell W. S., Martin J. G. The role of endogenous corticosterone in the late-phase response to allergen challenge in the brown Norway rat. Am J Respir Crit Care Med. 1996 Feb;153(2):545–550. doi: 10.1164/ajrccm.153.2.8564095. [DOI] [PubMed] [Google Scholar]
- Vianna E. O., Garcia-Leme J. Allergen-induced airway inflammation in rats. Role of insulin. Am J Respir Crit Care Med. 1995 Mar;151(3 Pt 1):809–814. doi: 10.1164/ajrccm/151.3_Pt_1.809. [DOI] [PubMed] [Google Scholar]
- Walls A. F., Rhee Y. K., Gould D. J., Walters C., Robinson C., Church M. K., Holgate S. T. Inflammatory mediators and cellular infiltration of the lungs in a guinea pig model of the late asthmatic reaction. Lung. 1991;169(4):227–240. doi: 10.1007/BF02714157. [DOI] [PubMed] [Google Scholar]
- Xu L. J., Eidelman D. H., Bates J. H., Martin J. G. Late response of the upper airway of the rat to inhaled antigen. J Appl Physiol (1985) 1990 Oct;69(4):1360–1365. doi: 10.1152/jappl.1990.69.4.1360. [DOI] [PubMed] [Google Scholar]