Abstract
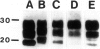
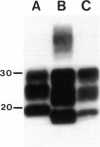
Antibodies to the prion protein (PrP) have been critical to the neuropathological and biochemical characterization of PrP-related degenerative diseases in humans and animals. Although PrP is highly conserved evolutionarily, there is some sequence divergence among species; as a consequence, anti-PrP antibodies have a wide spectrum of reactivity (from strong immunopositivity to lack of reactivity) when challenged with PrP from diverse species. We have produced an antibody (anti-PrP95-108) raised against a synthetic peptide corresponding to residues 95 to 108 of human PrP and have characterized it by epitope mapping, Western immunoblot analysis, and immunohistochemistry. The antibody recognizes not only human PrP isoforms but also pathological PrP from all species tested (ie, cattle, sheep, hamsters, and mice). This is probably due to the fact that the epitope recognized by this antibody includes residues 100 to 108 of human PrP, a sequence that is also present in PrP of several other species. Thus, this reagent is valuable not only for the study of human prion diseases but also for analysis of the possible relationship between human and animal disorders.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin M. A., Cohen F. E., Prusiner S. B. Prion protein isoforms, a convergence of biological and structural investigations. J Biol Chem. 1995 Aug 18;270(33):19197–19200. doi: 10.1074/jbc.270.33.19197. [DOI] [PubMed] [Google Scholar]
- Bolton D. C., Seligman S. J., Bablanian G., Windsor D., Scala L. J., Kim K. S., Chen C. M., Kascsak R. J., Bendheim P. E. Molecular location of a species-specific epitope on the hamster scrapie agent protein. J Virol. 1991 Jul;65(7):3667–3675. doi: 10.1128/jvi.65.7.3667-3675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G. A., Hsiao K., Oesch B., Westaway D., Prusiner S. B. Genetics of prion infections. Trends Genet. 1991 Feb;7(2):61–65. doi: 10.1016/0168-9525(91)90233-G. [DOI] [PubMed] [Google Scholar]
- Collinge J., Sidle K. C., Meads J., Ironside J., Hill A. F. Molecular analysis of prion strain variation and the aetiology of 'new variant' CJD. Nature. 1996 Oct 24;383(6602):685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann W., Hunter N., Foster J. D., Salbaum J. M., Beyreuther K., Hope J. Two alleles of a neural protein gene linked to scrapie in sheep. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2476–2480. doi: 10.1073/pnas.87.7.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann W., Hunter N., Martin T., Dawson M., Hope J. Different forms of the bovine PrP gene have five or six copies of a short, G-C-rich element within the protein-coding exon. J Gen Virol. 1991 Jan;72(Pt 1):201–204. doi: 10.1099/0022-1317-72-1-201. [DOI] [PubMed] [Google Scholar]
- Hill A. F., Desbruslais M., Joiner S., Sidle K. C., Gowland I., Collinge J., Doey L. J., Lantos P. The same prion strain causes vCJD and BSE. Nature. 1997 Oct 2;389(6650):448-50, 526. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Tonna-DeMasi M., Fersko R., Carp R. I., Wisniewski H. M., Diringer H. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol. 1987 Dec;61(12):3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Korth C., Stierli B., Streit P., Moser M., Schaller O., Fischer R., Schulz-Schaeffer W., Kretzschmar H., Raeber A., Braun U. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997 Nov 6;390(6655):74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H. A., Stowring L. E., Westaway D., Stubblebine W. H., Prusiner S. B., Dearmond S. J. Molecular cloning of a human prion protein cDNA. DNA. 1986 Aug;5(4):315–324. doi: 10.1089/dna.1986.5.315. [DOI] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Prusiner S. B. Prion protein genes: evolutionary and functional aspects. Curr Top Microbiol Immunol. 1991;172:109–124. doi: 10.1007/978-3-642-76540-7_7. [DOI] [PubMed] [Google Scholar]
- Parchi P., Castellani R., Capellari S., Ghetti B., Young K., Chen S. G., Farlow M., Dickson D. W., Sima A. A., Trojanowski J. Q. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 1996 Jun;39(6):767–778. doi: 10.1002/ana.410390613. [DOI] [PubMed] [Google Scholar]
- Piccardo P., Dagenais A., Cuello A. C., St-Pierre S., Nalbantoglu J. An antibody against the Alzheimer's disease amyloid precursor protein recognizes distinct conformational isoforms. Histochemistry. 1993 May;99(5):347–353. doi: 10.1007/BF00717046. [DOI] [PubMed] [Google Scholar]
- Piccardo P., Ghetti B., Dickson D. W., Vinters H. V., Giaccone G., Bugiani O., Tagliavini F., Young K., Dlouhy S. R., Seiler C. Gerstmann-Sträussler-Scheinker disease (PRNP P102L): amyloid deposits are best recognized by antibodies directed to epitopes in PrP region 90-165. J Neuropathol Exp Neurol. 1995 Nov;54(6):790–801. doi: 10.1097/00005072-199511000-00006. [DOI] [PubMed] [Google Scholar]
- Piccardo P., Seiler C., Dlouhy S. R., Young K., Farlow M. R., Prelli F., Frangione B., Bugiani O., Tagliavini F., Ghetti B. Proteinase-K-resistant prion protein isoforms in Gerstmann-Sträussler-Scheinker disease (Indiana kindred). J Neuropathol Exp Neurol. 1996 Nov;55(11):1157–1163. doi: 10.1097/00005072-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., McGrath H., Tam J. P. A novel method for producing anti-peptide antibodies. Production of site-specific antibodies to the T cell antigen receptor beta-chain. J Biol Chem. 1988 Feb 5;263(4):1719–1725. [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Sawh P. R., Wolfe G. C., Rubenstein R., Carp R. I., Innis M. A. Isolation of a cDNA clone encoding the leader peptide of prion protein and expression of the homologous gene in various tissues. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6377–6381. doi: 10.1073/pnas.83.17.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M., Serban D., Gyuris T., Scott M., Torchia T., Prusiner S. B. Epitope mapping of the Syrian hamster prion protein utilizing chimeric and mutant genes in a vaccinia virus expression system. J Immunol. 1991 Nov 15;147(10):3568–3574. [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest. 1991 May;64(5):693–702. [PubMed] [Google Scholar]
- Tagliavini F., McArthur R. A., Canciani B., Giaccone G., Porro M., Bugiani M., Lievens P. M., Bugiani O., Peri E., Dall'Ara P. Effectiveness of anthracycline against experimental prion disease in Syrian hamsters. Science. 1997 May 16;276(5315):1119–1122. doi: 10.1126/science.276.5315.1119. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Prelli F., Porro M., Rossi G., Giaccone G., Farlow M. R., Dlouhy S. R., Ghetti B., Bugiani O., Frangione B. Amyloid fibrils in Gerstmann-Sträussler-Scheinker disease (Indiana and Swedish kindreds) express only PrP peptides encoded by the mutant allele. Cell. 1994 Nov 18;79(4):695–703. doi: 10.1016/0092-8674(94)90554-1. [DOI] [PubMed] [Google Scholar]
- Westaway D., Goodman P. A., Mirenda C. A., McKinley M. P., Carlson G. A., Prusiner S. B. Distinct prion proteins in short and long scrapie incubation period mice. Cell. 1987 Nov 20;51(4):651–662. doi: 10.1016/0092-8674(87)90134-6. [DOI] [PubMed] [Google Scholar]