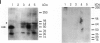Abstract
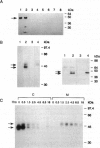
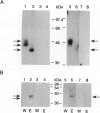
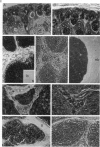
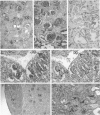
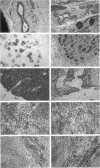
We previously established that the expression of the human nov gene (novH) was altered in Wilms' tumors and that levels of novH and WT1 mRNA were inversely correlated in individual Wilms' tumors. Insofar as novH has been shown to be a target for WT1 regulation, novH might play an important role during normal nephrogenesis and in the development of Wilms' tumors. We now show that during normal nephrogenesis novH protein is tightly associated with differentiation of glomerular podocytes. NovH expression is not restricted to renal differentiation but is also detected in endothelium and neural tissue of the kidney. Our results establish that alteration of novH expression in sporadic and heritable Wilms' tumors is associated with dysregulated expression of both novH mRNA and protein. In general, the highest novH expression was noted in the Wilms' tumor, genitourinary anomalies, aniridia, and mental retardation (WAGR)-associated Wilms' tumors. Expression in the Denys-Drash syndrome (DDS)-associated Wilms' tumors fell within the variable spectrum observed in sporadic Wilms' tumor cases. As in developing kidney podocytes, novH protein was also prominent in the abnormal hypoplastic podocytes from DDS cases and in kidney podocytes adjoining Wilms' tumors. In Wilms' tumors exhibiting heterotypic differentiation, novH protein was expressed at high levels in tumor-derived striated muscle and at lower levels in tumor-derived cartilage. These observations taken together indicate that novH may represent both a marker of podocytic differentiation in kidney and a marker of heterotypic mesenchymal differentiation in Wilms' tumors. In addition, absence or very low levels of WT1 are correlated with higher novH expression, and its variable expression in cases with mutant WT1 (sporadic and DDS) suggests that the potential activation and repression transcriptional functions possessed by WT1 are likely dependent on the specific mutation incurred.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird P. N., Groves N., Haber D. A., Housman D. E., Cowell J. K. Identification of mutations in the WT1 gene in tumours from patients with the WAGR syndrome. Oncogene. 1992 Nov;7(11):2141–2149. [PubMed] [Google Scholar]
- Beckwith J. B., Kiviat N. B., Bonadio J. F. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Pediatr Pathol. 1990;10(1-2):1–36. doi: 10.3109/15513819009067094. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993 Jul 26;327(2):125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- Bradham D. M., Igarashi A., Potter R. L., Grotendorst G. R. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991 Sep;114(6):1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow N. E., Langholz B. Childhood cancer incidence: geographical and temporal variations. Int J Cancer. 1983 Dec 15;32(6):703–716. doi: 10.1002/ijc.2910320609. [DOI] [PubMed] [Google Scholar]
- Breslow N., Beckwith J. B., Ciol M., Sharples K. Age distribution of Wilms' tumor: report from the National Wilms' Tumor Study. Cancer Res. 1988 Mar 15;48(6):1653–1657. [PubMed] [Google Scholar]
- Brodie S. G., Stocker S. J., Wardlaw J. C., Duncan M. H., McConnell T. S., Feddersen R. M., Williams T. M. EWS and WT-1 gene fusion in desmoplastic small round cell tumor of the abdomen. Hum Pathol. 1995 Dec;26(12):1370–1374. doi: 10.1016/0046-8177(95)90303-8. [DOI] [PubMed] [Google Scholar]
- Catzavelos C., Bhattacharya N., Ung Y. C., Wilson J. A., Roncari L., Sandhu C., Shaw P., Yeger H., Morava-Protzner I., Kapusta L. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997 Feb;3(2):227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Perbal B. Altérations génétiques associées à la différenciation pathologique des tumeurs de Wilms. Bull Cancer. 1997 Mar;84(3):289–303. [PubMed] [Google Scholar]
- Coppes M. J., Clericuzio C. L. "Molecular genetic analysis of the WT1 gene in patients suspected to have the Denys-Drash syndrome". Med Pediatr Oncol. 1994;23(4):390–390. doi: 10.1002/mpo.2950230414. [DOI] [PubMed] [Google Scholar]
- Coppes M. J., Liefers G. J., Paul P., Yeger H., Williams B. R. Homozygous somatic Wt1 point mutations in sporadic unilateral Wilms tumor. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1416–1419. doi: 10.1073/pnas.90.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Gansler T., Allen K. D., Burant C. F., Inabnett T., Scott A., Buse M. G., Sens D. A., Garvin A. J. Detection of type 1 insulinlike growth factor (IGF) receptors in Wilms' tumors. Am J Pathol. 1988 Mar;130(3):431–435. [PMC free article] [PubMed] [Google Scholar]
- Grotendorst G. R., Okochi H., Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996 Apr;7(4):469–480. [PubMed] [Google Scholar]
- Grubb G. R., Yun K., Williams B. R., Eccles M. R., Reeve A. E. Expression of WT1 protein in fetal kidneys and Wilms tumors. Lab Invest. 1994 Oct;71(4):472–479. [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Haselbacher G. K., Irminger J. C., Zapf J., Ziegler W. H., Humbel R. E. Insulin-like growth factor II in human adrenal pheochromocytomas and Wilms tumors: expression at the mRNA and protein level. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1104–1106. doi: 10.1073/pnas.84.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D. The genetics of Wilms' tumor--a case of disrupted development. Annu Rev Genet. 1994;28:523–558. doi: 10.1146/annurev.ge.28.120194.002515. [DOI] [PubMed] [Google Scholar]
- Johnstone R. W., See R. H., Sells S. F., Wang J., Muthukkumar S., Englert C., Haber D. A., Licht J. D., Sugrue S. P., Roberts T. A novel repressor, par-4, modulates transcription and growth suppression functions of the Wilms' tumor suppressor WT1. Mol Cell Biol. 1996 Dec;16(12):6945–6956. doi: 10.1128/mcb.16.12.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot V., Martinerie C., Dambrine G., Plassiart G., Brisac M., Crochet J., Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992 Jan;12(1):10–21. doi: 10.1128/mcb.12.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Nagalla S. R., Oh Y., Wilson E., Roberts C. T., Jr, Rosenfeld R. G. Identification of a family of low-affinity insulin-like growth factor binding proteins (IGFBPs): characterization of connective tissue growth factor as a member of the IGFBP superfamily. Proc Natl Acad Sci U S A. 1997 Nov 25;94(24):12981–12986. doi: 10.1073/pnas.94.24.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Yeger H., Han R., Wallace M., Goldstein B., Rotin D. Expression of LAR-PTP2 in rat lung is confined to proliferating epithelia lining the airways and air sacs. Am J Physiol. 1996 Apr;270(4 Pt 1):L566–L576. doi: 10.1152/ajplung.1996.270.4.L566. [DOI] [PubMed] [Google Scholar]
- Kireeva M. L., MO F. E., Yang G. P., Lau L. F. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996 Apr;16(4):1326–1334. doi: 10.1128/mcb.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Ladanyi M., Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994 Jun 1;54(11):2837–2840. [PubMed] [Google Scholar]
- Lahoti C., Thorner P., Malkin D., Yeger H. Immunohistochemical detection of p53 in Wilms' tumors correlates with unfavorable outcome. Am J Pathol. 1996 May;148(5):1577–1589. [PMC free article] [PubMed] [Google Scholar]
- Louvard D. Apical membrane aminopeptidase appears at site of cell-cell contact in cultured kidney epithelial cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4132–4136. doi: 10.1073/pnas.77.7.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S., Englert C., Bennett P., Heinrich G., Haber D. A. The WT1 gene product stabilizes p53 and inhibits p53-mediated apoptosis. Genes Dev. 1995 Sep 1;9(17):2143–2156. doi: 10.1101/gad.9.17.2143. [DOI] [PubMed] [Google Scholar]
- Maheswaran S., Park S., Bernard A., Morris J. F., Rauscher F. J., 3rd, Hill D. E., Haber D. A. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5100–5104. doi: 10.1073/pnas.90.11.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinerie C., Chevalier G., Rauscher F. J., 3rd, Perbal B. Regulation of nov by WT1: a potential role for nov in nephrogenesis. Oncogene. 1996 Apr 4;12(7):1479–1492. [PubMed] [Google Scholar]
- Martinerie C., Huff V., Joubert I., Badzioch M., Saunders G., Strong L., Perbal B. Structural analysis of the human nov proto-oncogene and expression in Wilms tumor. Oncogene. 1994 Sep;9(9):2729–2732. [PubMed] [Google Scholar]
- Matsunaga E. Genetics of Wilms' tumor. Hum Genet. 1981;57(3):231–246. doi: 10.1007/BF00278936. [DOI] [PubMed] [Google Scholar]
- Maw M. A., Grundy P. E., Millow L. J., Eccles M. R., Dunn R. S., Smith P. J., Feinberg A. P., Law D. J., Paterson M. C., Telzerow P. E. A third Wilms' tumor locus on chromosome 16q. Cancer Res. 1992 Jun 1;52(11):3094–3098. [PubMed] [Google Scholar]
- Mundlos S., Pelletier J., Darveau A., Bachmann M., Winterpacht A., Zabel B. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development. 1993 Dec;119(4):1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- Newsham I., Kindler-Röhrborn A., Daub D., Cavenee W. A constitutional BWS-related t(11;16) chromosome translocation occurring in the same region of chromosome 16 implicated in Wilms' tumors. Genes Chromosomes Cancer. 1995 Jan;12(1):1–7. doi: 10.1002/gcc.2870120102. [DOI] [PubMed] [Google Scholar]
- O'Brien T. P., Lau L. F. Expression of the growth factor-inducible immediate early gene cyr61 correlates with chondrogenesis during mouse embryonic development. Cell Growth Differ. 1992 Sep;3(9):645–654. [PubMed] [Google Scholar]
- O'Brien T. P., Yang G. P., Sanders L., Lau L. F. Expression of cyr61, a growth factor-inducible immediate-early gene. Mol Cell Biol. 1990 Jul;10(7):3569–3577. doi: 10.1128/mcb.10.7.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Ouchida M., Lee L., Gatalica Z., Rao V. N., Reddy E. S. The EWS gene, involved in Ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene. 1994 Oct;9(10):3087–3097. [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Patwardhan S., Gashler A., Siegel M. G., Chang L. C., Joseph L. J., Shows T. B., Le Beau M. M., Sukhatme V. P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991 Jun;6(6):917–928. [PubMed] [Google Scholar]
- Peier A. M., Meloni A. M., Erling M. A., Sandberg A. A. Involvement of chromosome 7 in Wilms tumor. Cancer Genet Cytogenet. 1995 Jan;79(1):92–94. doi: 10.1016/0165-4608(94)00110-w. [DOI] [PubMed] [Google Scholar]
- Pelletier J. Molecular genetics of Wilms' tumor: insights into normal and abnormal renal development. Can J Oncol. 1994 Apr;4(2):262–272. [PubMed] [Google Scholar]
- Perbal B. Contribution of MAV-1-induced nephroblastoma to the study of genes involved in human Wilms' tumor development. Crit Rev Oncog. 1994;5(6):589–613. [PubMed] [Google Scholar]
- Radice P., Perotti D., De Benedetti V., Mondini P., Radice M. T., Pilotti S., Luksch R., Fossati Bellani F., Pierotti M. A. Allelotyping in Wilms tumors identifies a putative third tumor suppressor gene on chromosome 11. Genomics. 1995 Jun 10;27(3):497–501. doi: 10.1006/geno.1995.1082. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Eccles M. R., Wilkins R. J., Bell G. I., Millow L. J. Expression of insulin-like growth factor-II transcripts in Wilms' tumour. Nature. 1985 Sep 19;317(6034):258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Macdonald-Bravo H., Mattéi M. G., Bravo R. Structure, mapping, and expression of fisp-12, a growth factor-inducible gene encoding a secreted cysteine-rich protein. Cell Growth Differ. 1991 May;2(5):225–233. [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz G., Martinerie C., Perbal B., Hanafusa H. Transcriptional down regulation of the nov proto-oncogene in fibroblasts transformed by p60v-src. Mol Cell Biol. 1996 Feb;16(2):481–486. doi: 10.1128/mcb.16.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton C. C., Huff V., Call K. M., Cohn S., Strong L. C., Housman D. E., Saunders G. F. Smallest region of overlap in Wilms tumor deletions uniquely implicates an 11p13 zinc finger gene as the disease locus. Genomics. 1991 May;10(1):293–297. doi: 10.1016/0888-7543(91)90516-h. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y., Qiu Q. Q., Gurrieri M., Huang J., Deuel T. F. WT1, the Wilms' tumor suppressor gene product, represses transcription through an interactive nuclear protein. Oncogene. 1995 Mar 16;10(6):1243–1247. [PubMed] [Google Scholar]
- Werner H., Rauscher F. J., 3rd, Sukhatme V. P., Drummond I. A., Roberts C. T., Jr, LeRoith D. Transcriptional repression of the insulin-like growth factor I receptor (IGF-I-R) gene by the tumor suppressor WT1 involves binding to sequences both upstream and downstream of the IGF-I-R gene transcription start site. J Biol Chem. 1994 Apr 29;269(17):12577–12582. [PubMed] [Google Scholar]
- Werner H., Re G. G., Drummond I. A., Sukhatme V. P., Rauscher F. J., 3rd, Sens D. A., Garvin A. J., LeRoith D., Roberts C. T., Jr Increased expression of the insulin-like growth factor I receptor gene, IGF1R, in Wilms tumor is correlated with modulation of IGF1R promoter activity by the WT1 Wilms tumor gene product. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5828–5832. doi: 10.1073/pnas.90.12.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. P., Lau L. F. Cyr61, product of a growth factor-inducible immediate early gene, is associated with the extracellular matrix and the cell surface. Cell Growth Differ. 1991 Jul;2(7):351–357. [PubMed] [Google Scholar]
- Ye Y., Raychaudhuri B., Gurney A., Campbell C. E., Williams B. R. Regulation of WT1 by phosphorylation: inhibition of DNA binding, alteration of transcriptional activity and cellular translocation. EMBO J. 1996 Oct 15;15(20):5606–5615. [PMC free article] [PubMed] [Google Scholar]
- Yeger H., Cullinane C., Flenniken A., Chilton-MacNeil S., Campbell C., Huang A., Bonetta L., Coppes M. J., Thorner P., Williams B. R. Coordinate expression of Wilms' tumor genes correlates with Wilms' tumor phenotypes. Cell Growth Differ. 1992 Dec;3(12):855–864. [PubMed] [Google Scholar]
- Yeger H., Forget D., Alami J., Williams B. R. Analysis of WT1 gene expression during mouse nephrogenesis in organ culture. In Vitro Cell Dev Biol Anim. 1996 Sep;32(8):496–504. doi: 10.1007/BF02723053. [DOI] [PubMed] [Google Scholar]