Abstract
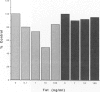
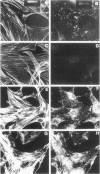
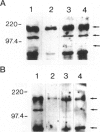
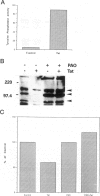
HIV-1 Tat plays a role in the pathogenesis of Kaposi's sarcoma. We therefore investigated the effect of Tat on the growth of murine Kaposi's sarcoma-like spindle (TTB) cells derived from dermal lesions. We observed that Tat and a peptide corresponding to the carboxyl-terminal region (Tat65-80) containing an RGD sequence inhibit TTB cell proliferation only when cells are cultured on fibronectin. This inhibitory effect correlates with redistribution of the alpha(v) integrin subunit on the surface of TTB cells and with down-regulation of tyrosine phosphorylation of specific substrates due to an increased tyrosine phosphatase activity. Indeed, phenylarsine oxide, a potent inhibitor of phosphotyrosine phosphatases, prevented the effects of Tat on TTB cells. We therefore argue that the action of Tat on TTB cells is mediated by the RGD motif through an integrin-based cell signaling pathway involving the activity of phosphotyrosine phosphatase(s), which would lead to a decrease in the levels of phosphotyrosine-containing proteins, among which is erk-2/p42MAPK.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Barillari G., Benelli R., Gallo R. C., Ensoli B. Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4838–4842. doi: 10.1073/pnas.92.11.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A., Soldi R., Giunciuglio D., Giraudo E., Benelli R., Primo L., Noonan D., Salio M., Camussi G., Rockl W. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996 Dec;2(12):1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- Amaral M. C., Miles S., Kumar G., Nel A. E. Oncostatin-M stimulates tyrosine protein phosphorylation in parallel with the activation of p42MAPK/ERK-2 in Kaposi's cells. Evidence that this pathway is important in Kaposi cell growth. J Clin Invest. 1993 Aug;92(2):848–857. doi: 10.1172/JCI116659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo A. G., Campanero M. R., Sánchez-Mateos P., Zapata J. M., Ursa M. A., del Pozo M. A., Sánchez-Madrid F. Induction of tyrosine phosphorylation during ICAM-3 and LFA-1-mediated intercellular adhesion, and its regulation by the CD45 tyrosine phosphatase. J Cell Biol. 1994 Sep;126(5):1277–1286. doi: 10.1083/jcb.126.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillari G., Gendelman R., Gallo R. C., Ensoli B. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer K. J., Johnson G. L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994 Jun;19(6):236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Debouck C., Biesecker G. Identification of an Arg-Gly-Asp (RGD) cell adhesion site in human immunodeficiency virus type 1 transactivation protein, tat. J Cell Biol. 1990 Sep;111(3):1275–1281. doi: 10.1083/jcb.111.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L., Barillari G., Chang H. K., Bohan C. A., Kao V., Morgan R., Gallo R. C., Ensoli B. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J Virol. 1992 Dec;66(12):7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cavallaro U., Gasparini G., Soria M. R., Maier J. A. Spindle cells isolated from Kaposi's sarcoma-like lesions of BKV/tat-transgenic mice co-express markers of different cell types. AIDS. 1996 Sep;10(11):1211–1219. doi: 10.1097/00002030-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Cavallaro U., Mariotti M., Wu Z. H., Soria M. R., Maier J. A. Fibronectin modulates endothelial response to HIV type 1 Tat. AIDS Res Hum Retroviruses. 1997 Oct 10;13(15):1341–1348. doi: 10.1089/aid.1997.13.1341. [DOI] [PubMed] [Google Scholar]
- Chang Y., Cesarman E., Pessin M. S., Lee F., Culpepper J., Knowles D. M., Moore P. S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994 Dec 16;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Corallini A., Altavilla G., Pozzi L., Bignozzi F., Negrini M., Rimessi P., Gualandi F., Barbanti-Brodano G. Systemic expression of HIV-1 tat gene in transgenic mice induces endothelial proliferation and tumors of different histotypes. Cancer Res. 1993 Nov 15;53(22):5569–5575. [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R. A., Wingfield P., Gallo R. C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993 Jan;67(1):277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Gendelman R., Markham P., Fiorelli V., Colombini S., Raffeld M., Cafaro A., Chang H. K., Brady J. N., Gallo R. C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature. 1994 Oct 20;371(6499):674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995 Jan 27;80(2):225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Liao K., Hoffman R. D., Lane M. D. Phosphotyrosyl turnover in insulin signaling. Characterization of two membrane-bound pp15 protein tyrosine phosphatases from 3T3-L1 adipocytes. J Biol Chem. 1991 Apr 5;266(10):6544–6553. [PubMed] [Google Scholar]
- Lotz M., Clark-Lewis I., Ganu V. HIV-1 transactivator protein Tat induces proliferation and TGF beta expression in human articular chondrocytes. J Cell Biol. 1994 Feb;124(3):365–371. doi: 10.1083/jcb.124.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. A. Activation of phosphotyrosine phosphatase activity by reduction of cell-substrate adhesion. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11177–11181. doi: 10.1073/pnas.90.23.11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Naldini L., Primavera M. V., Teti A., Zambonin-Zallone A. Cell-substratum interaction of cultured avian osteoclasts is mediated by specific adhesion structures. J Cell Biol. 1984 Nov;99(5):1696–1705. doi: 10.1083/jcb.99.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon A., Leoni C., Benfenati F., Valtorta F. Tat protein from HIV-1 activates MAP kinase in granular neurons and glial cells from rat cerebellum. Biochem Biophys Res Commun. 1997 Sep 29;238(3):800–805. doi: 10.1006/bbrc.1997.7393. [DOI] [PubMed] [Google Scholar]
- Morgavi P., Bonifaci N., Pagani M., Costigliolo S., Sitia R., Rubartelli A. The association of HIV-1 Tat with nuclei is regulated by Ca2+ ions and cytosolic factors. J Biol Chem. 1997 Apr 25;272(17):11256–11260. doi: 10.1074/jbc.272.17.11256. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Salahuddin S. Z., Biberfeld P., Ensoli B., Markham P. D., Wong-Staal F., Gallo R. C. Kaposi's sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science. 1988 Oct 21;242(4877):426–430. doi: 10.1126/science.3262925. [DOI] [PubMed] [Google Scholar]
- Reuter C. W., Catling A. D., Jelinek T., Weber M. J. Biochemical analysis of MEK activation in NIH3T3 fibroblasts. Identification of B-Raf and other activators. J Biol Chem. 1995 Mar 31;270(13):7644–7655. doi: 10.1074/jbc.270.13.7644. [DOI] [PubMed] [Google Scholar]
- Rusciano D., Lorenzoni P., Burger M. M. Constitutive activation of c-Met in liver metastatic B16 melanoma cells depends on both substrate adhesion and cell density and is regulated by a cytosolic tyrosine phosphatase activity. J Biol Chem. 1996 Aug 23;271(34):20763–20769. doi: 10.1074/jbc.271.34.20763. [DOI] [PubMed] [Google Scholar]
- Scala G., Ruocco M. R., Ambrosino C., Mallardo M., Giordano V., Baldassarre F., Dragonetti E., Quinto I., Venuta S. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. J Exp Med. 1994 Mar 1;179(3):961–971. doi: 10.1084/jem.179.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem. 1996 Jan 12;271(2):603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- Vaishnav Y. N., Wong-Staal F. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- Westendorp M. O., Frank R., Ochsenbauer C., Stricker K., Dhein J., Walczak H., Debatin K. M., Krammer P. H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995 Jun 8;375(6531):497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]