Abstract
Oxidatively damaged LDL may be of central importance in atherogenesis. Epidemiological evidence suggests that high dietary intakes of beta-carotene and vitamin E decreases the risk for atherosclerotic vascular disease, raising the possibility that lipid-soluble antioxidants slow vascular disease by protecting LDL from oxidation. To test this hypothesis, we fed male New Zealand White rabbits a high-cholesterol diet or the same diet supplemented with either 1% probucol, 0.01% vitamin E, 0.01% all-trans beta-carotene, or 0.01% 9-cis beta-carotene; then we assessed both the susceptibility of LDL to oxidation ex vivo and the extent of aortic atherosclerosis. As in earlier studies, probucol protected LDL from oxidation and inhibited lesion formation. In contrast, vitamin E modestly inhibited LDL oxidation but did not prevent atherosclerosis. While beta-carotene had no effect on LDL oxidation ex vivo, the all-trans isomer inhibited lesion formation to the same degree as probucol. Moreover, all-trans beta-carotene was undetectable in LDL isolated from rabbits fed the compound, although tissue levels of retinyl palmitate were increased. The effect of all-trans beta-carotene on atherogenesis can thus be separated from the resistance of LDL to oxidation, indicating that other mechanisms may account for the ability of this compound to prevent vascular disease. Our results suggest that metabolites derived from all-trans beta-carotene inhibit atherosclerosis in hypercholesterolemic rabbits, possibly via stereospecific interactions with retinoic acid receptors in the artery wall.
Full text
PDF

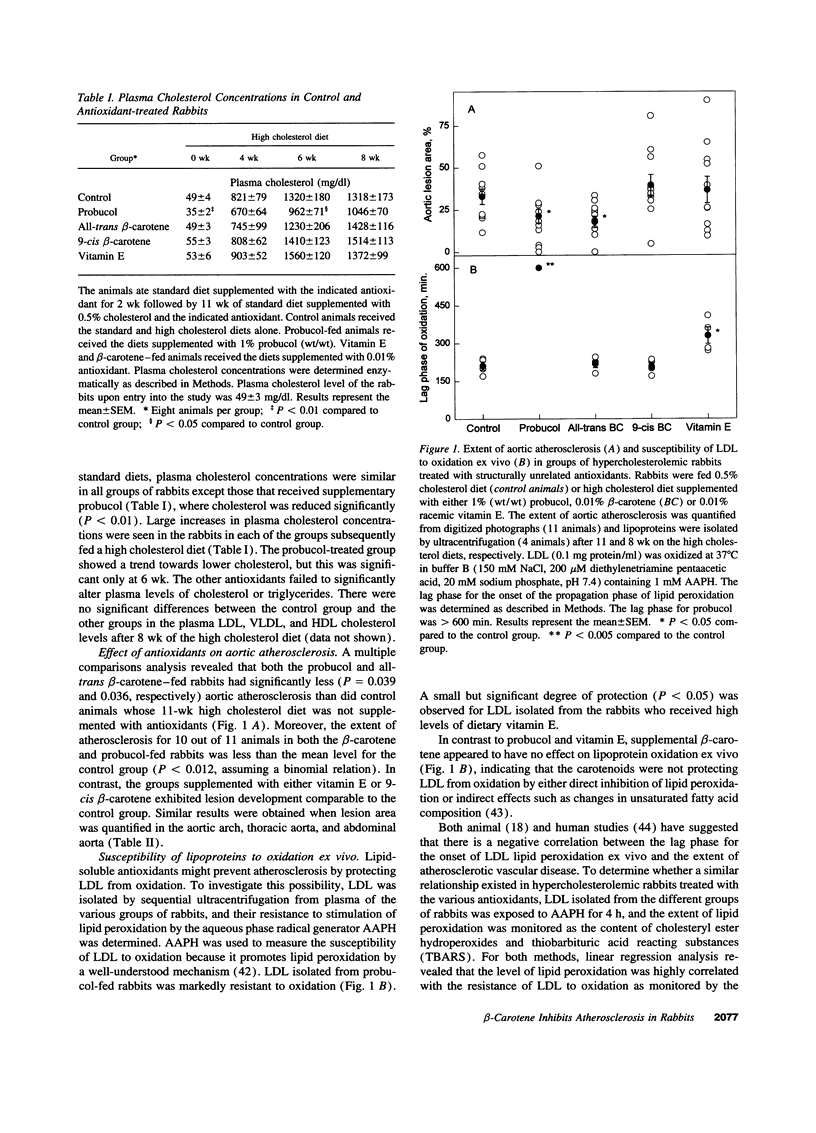
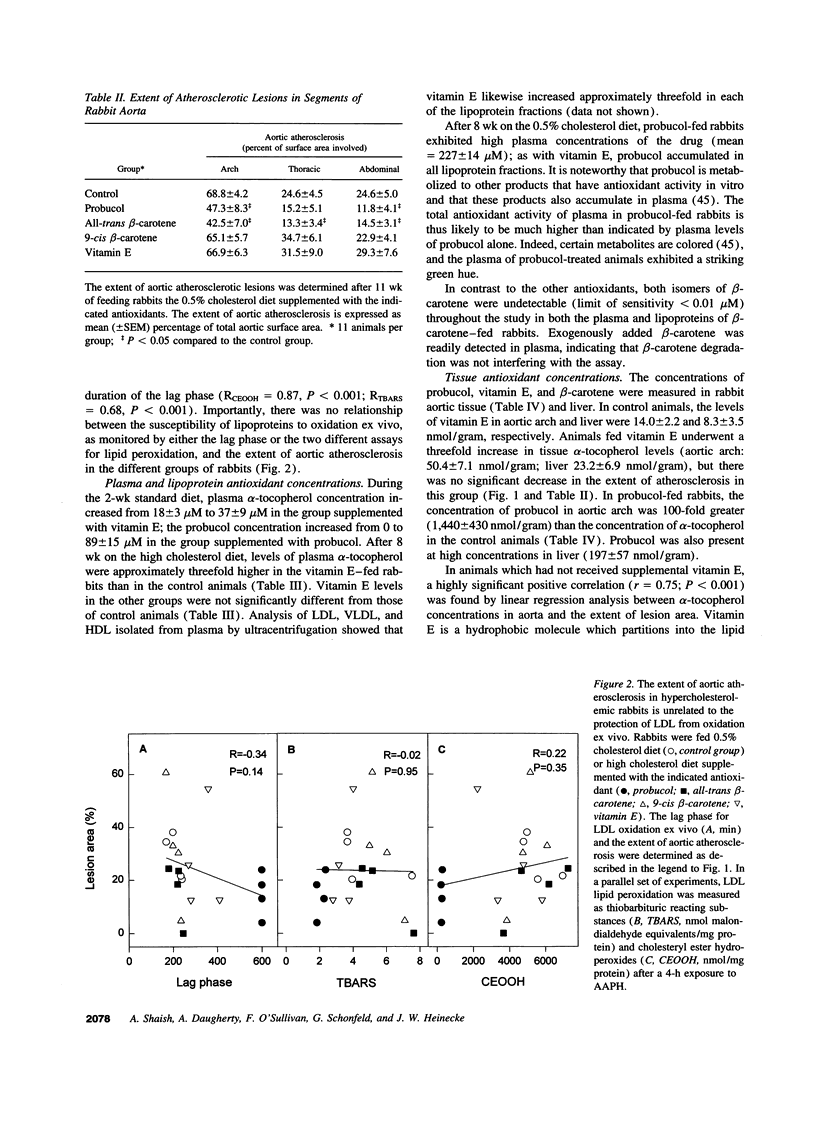
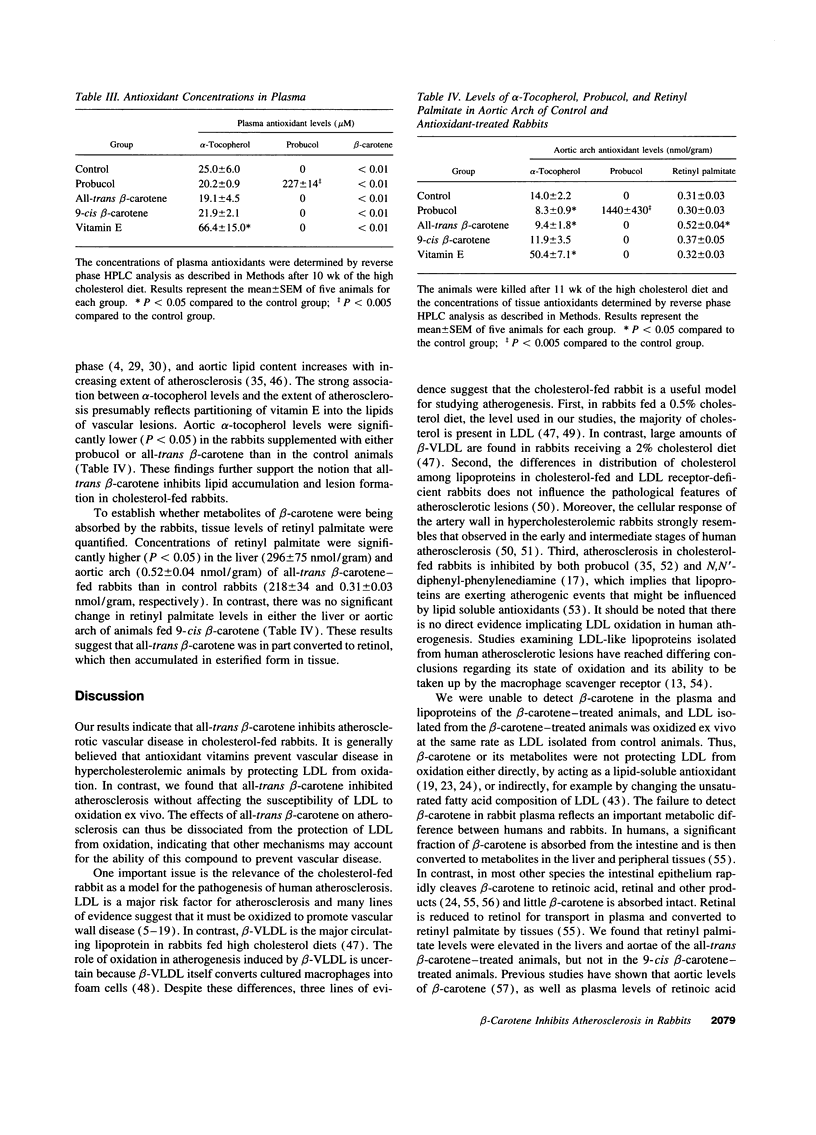



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart R. L., Busch S. J., Jackson R. L. Concentration-dependent antioxidant activity of probucol in low density lipoproteins in vitro: probucol degradation precedes lipoprotein oxidation. J Lipid Res. 1989 Nov;30(11):1703–1710. [PubMed] [Google Scholar]
- Ben-Amotz A., Lers A., Avron M. Stereoisomers of beta-Carotene and Phytoene in the Alga Dunaliella bardawil. Plant Physiol. 1988 Apr;86(4):1286–1291. doi: 10.1104/pp.86.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson L. K., Kayden H. J., Miller E., Moshell A. N. The transport of alpha-tocopherol and beta-carotene in human blood. J Lipid Res. 1976 Jul;17(4):343–352. [PubMed] [Google Scholar]
- Björkhem I., Henriksson-Freyschuss A., Breuer O., Diczfalusy U., Berglund L., Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991 Jan-Feb;11(1):15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Green M. H., Berg T., Norum K. R. Transport and storage of vitamin A. Science. 1990 Oct 19;250(4979):399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- Bocan T. M., Mueller S. B., Mazur M. J., Uhlendorf P. D., Brown E. Q., Kieft K. A. The relationship between the degree of dietary-induced hypercholesterolemia in the rabbit and atherosclerotic lesion formation. Atherosclerosis. 1993 Aug;102(1):9–22. doi: 10.1016/0021-9150(93)90080-e. [DOI] [PubMed] [Google Scholar]
- Burn T. C., Petrovick M. S., Hohaus S., Rollins B. J., Tenen D. G. Monocyte chemoattractant protein-1 gene is expressed in activated neutrophils and retinoic acid-induced human myeloid cell lines. Blood. 1994 Oct 15;84(8):2776–2783. [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Rateri D. L. Presence of LDL receptor-related protein/alpha 2-macroglobulin receptors in macrophages of atherosclerotic lesions from cholesterol-fed New Zealand and heterozygous Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1994 Dec;14(12):2017–2024. doi: 10.1161/01.atv.14.12.2017. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Roselaar S. E. Lipoprotein oxidation as a mediator of atherogenesis: insights from pharmacological studies. Cardiovasc Res. 1995 Mar;29(3):297–311. [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Schonfeld G. Probucol attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Br J Pharmacol. 1989 Oct;98(2):612–618. doi: 10.1111/j.1476-5381.1989.tb12635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Schonfeld G. The effects of probucol on the progression of atherosclerosis in mature Watanabe heritable hyperlipidaemic rabbits. Br J Pharmacol. 1991 May;103(1):1013–1018. doi: 10.1111/j.1476-5381.1991.tb12293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Sobel B. E., Schonfeld G. Isolation of low density lipoprotein from atherosclerotic vascular tissue of Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis. 1988 Nov-Dec;8(6):768–777. doi: 10.1161/01.atv.8.6.768. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Folman Y., Russell R. M., Tang G. W., Wolf D. G. Rabbits fed on beta-carotene have higher serum levels of all-trans retinoic acid than those receiving no beta-carotene. Br J Nutr. 1989 Jul;62(1):195–201. doi: 10.1079/bjn19890019. [DOI] [PubMed] [Google Scholar]
- Fruebis J., Steinberg D., Dresel H. A., Carew T. E. A comparison of the antiatherogenic effects of probucol and of a structural analogue of probucol in low density lipoprotein receptor-deficient rabbits. J Clin Invest. 1994 Jul;94(1):392–398. doi: 10.1172/JCI117334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Hajduczok Z. D., Weiss R. M., Stanford W., Marcus M. L. Determination of right ventricular mass in humans and dogs with ultrafast cardiac computed tomography. Circulation. 1990 Jul;82(1):202–212. doi: 10.1161/01.cir.82.1.202. [DOI] [PubMed] [Google Scholar]
- Hara S., Nagano Y., Sasada M., Kita T. Probucol pretreatment enhances the chemotaxis of mouse peritoneal macrophages. Arterioscler Thromb. 1992 May;12(5):593–600. doi: 10.1161/01.atv.12.5.593. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Horie S., Kizaki K., Kazama M. Retinoic acid counteracts both the downregulation of thrombomodulin and the induction of tissue factor in cultured human endothelial cells exposed to tumor necrosis factor. Blood. 1992 Nov 15;80(10):2556–2562. [PubMed] [Google Scholar]
- Jialal I., Norkus E. P., Cristol L., Grundy S. M. beta-Carotene inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1991 Oct 15;1086(1):134–138. doi: 10.1016/0005-2760(91)90164-d. [DOI] [PubMed] [Google Scholar]
- Keaney J. F., Jr, Gaziano J. M., Xu A., Frei B., Curran-Celentano J., Shwaery G. T., Loscalzo J., Vita J. A. Dietary antioxidants preserve endothelium-dependent vessel relaxation in cholesterol-fed rabbits. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11880–11884. doi: 10.1073/pnas.90.24.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinveld H. A., Demacker P. N., Stalenhoef A. F. Comparative study on the effect of low-dose vitamin E and probucol on the susceptibility of LDL to oxidation and the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1994 Aug;14(8):1386–1391. doi: 10.1161/01.atv.14.8.1386. [DOI] [PubMed] [Google Scholar]
- Lavy A., Ben Amotz A., Aviram M. Preferential inhibition of LDL oxidation by the all-trans isomer of beta-carotene in comparison with 9-cis beta-carotene. Eur J Clin Chem Clin Biochem. 1993 Feb;31(2):83–90. doi: 10.1515/cclm.1993.31.2.83. [DOI] [PubMed] [Google Scholar]
- Leid M., Kastner P., Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992 Oct;17(10):427–433. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- Mahley R. W. Development of accelerated atherosclerosis. Concepts derived from cell biology and animal model studies. Arch Pathol Lab Med. 1983 Aug;107(8):393–399. [PubMed] [Google Scholar]
- Mangelsdorf D. J., Kliewer S. A., Kakizuka A., Umesono K., Evans R. M. Retinoid receptors. Recent Prog Horm Res. 1993;48:99–121. doi: 10.1016/b978-0-12-571148-7.50008-7. [DOI] [PubMed] [Google Scholar]
- Mao S. J., Yates M. T., Parker R. A., Chi E. M., Jackson R. L. Attenuation of atherosclerosis in a modified strain of hypercholesterolemic Watanabe rabbits with use of a probucol analogue (MDL 29,311) that does not lower serum cholesterol. Arterioscler Thromb. 1991 Sep-Oct;11(5):1266–1275. doi: 10.1161/01.atv.11.5.1266. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Thomas C. E., Weintraub B., Hagaman K. A. Modulation of the physical state of cellular cholesteryl esters by 4,4'-(isopropylidenedithio)bis(2,6-di-t-butylphenol) (probucol). J Biol Chem. 1992 Jun 15;267(17):12291–12298. [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki E., Yamamoto Y., Komuro E., Sato K. Membrane damage due to lipid oxidation. Am J Clin Nutr. 1991 Jan;53(1 Suppl):201S–205S. doi: 10.1093/ajcn/53.1.201S. [DOI] [PubMed] [Google Scholar]
- O'Brien K., Nagano Y., Gown A., Kita T., Chait A. Probucol treatment affects the cellular composition but not anti-oxidized low density lipoprotein immunoreactivity of plaques from Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):751–759. doi: 10.1161/01.atv.11.3.751. [DOI] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palozza P., Krinsky N. I. Antioxidant effects of carotenoids in vivo and in vitro: an overview. Methods Enzymol. 1992;213:403–420. doi: 10.1016/0076-6879(92)13142-k. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S. Evidence for an additional intracellular site of action of probucol in the prevention of oxidative modification of low density lipoprotein. Use of a new water-soluble probucol derivative. J Clin Invest. 1992 May;89(5):1618–1621. doi: 10.1172/JCI115757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., van Poppel G., Vogelezang C., Buytenhek R., Kok F. J. Supplementation with vitamin E but not beta-carotene in vivo protects low density lipoprotein from lipid peroxidation in vitro. Effect of cigarette smoking. Arterioscler Thromb. 1992 May;12(5):554–562. doi: 10.1161/01.atv.12.5.554. [DOI] [PubMed] [Google Scholar]
- Reaven P. D., Khouw A., Beltz W. F., Parthasarathy S., Witztum J. L. Effect of dietary antioxidant combinations in humans. Protection of LDL by vitamin E but not by beta-carotene. Arterioscler Thromb. 1993 Apr;13(4):590–600. doi: 10.1161/01.atv.13.4.590. [DOI] [PubMed] [Google Scholar]
- Regnström J., Nilsson J., Tornvall P., Landou C., Hamsten A. Susceptibility to low-density lipoprotein oxidation and coronary atherosclerosis in man. Lancet. 1992 May 16;339(8803):1183–1186. doi: 10.1016/0140-6736(92)91129-v. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Chait A., Bierman E. L., King W., Goodwin P., Walden C. E., Ross R. Lipid composition of aorta of Watanabe heritable hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Plasma lipid composition determines aortic lipid composition of hypercholesterolemic rabbits. Arteriosclerosis. 1988 Jul-Aug;8(4):338–347. doi: 10.1161/01.atv.8.4.338. [DOI] [PubMed] [Google Scholar]
- Sasahara M., Raines E. W., Chait A., Carew T. E., Steinberg D., Wahl P. W., Ross R. Inhibition of hypercholesterolemia-induced atherosclerosis in the nonhuman primate by probucol. I. Is the extent of atherosclerosis related to resistance of LDL to oxidation? J Clin Invest. 1994 Jul;94(1):155–164. doi: 10.1172/JCI117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savenkova M. L., Mueller D. M., Heinecke J. W. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem. 1994 Aug 12;269(32):20394–20400. [PubMed] [Google Scholar]
- Small D. M. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988 Mar-Apr;8(2):103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Doebber T. W., Olszewski J., Wu M. S., Ventre J., Stevens K. A., Chao Y. S. Low density lipoprotein is protected from oxidation and the progression of atherosclerosis is slowed in cholesterol-fed rabbits by the antioxidant N,N'-diphenyl-phenylenediamine. J Clin Invest. 1992 Jun;89(6):1885–1891. doi: 10.1172/JCI115793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M. J., Hennekens C. H., Manson J. E., Colditz G. A., Rosner B., Willett W. C. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993 May 20;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Stary H. C., Blankenhorn D. H., Chandler A. B., Glagov S., Insull W., Jr, Richardson M., Rosenfeld M. E., Schaffer S. A., Schwartz C. J., Wagner W. D. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb. 1992 Jan;12(1):120–134. doi: 10.1161/01.atv.12.1.120. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Lougheed M. Scavenger receptor-independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arterioscler Thromb. 1992 May;12(5):608–625. doi: 10.1161/01.atv.12.5.608. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. J., Thornburg T., Manning J., Hooper K., Rudel L. L. Fatty acid composition of low-density lipoprotein influences its susceptibility to autoxidation. Biochemistry. 1994 Feb 22;33(7):1828–1834. doi: 10.1021/bi00173a028. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]


