Abstract
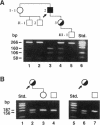
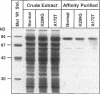
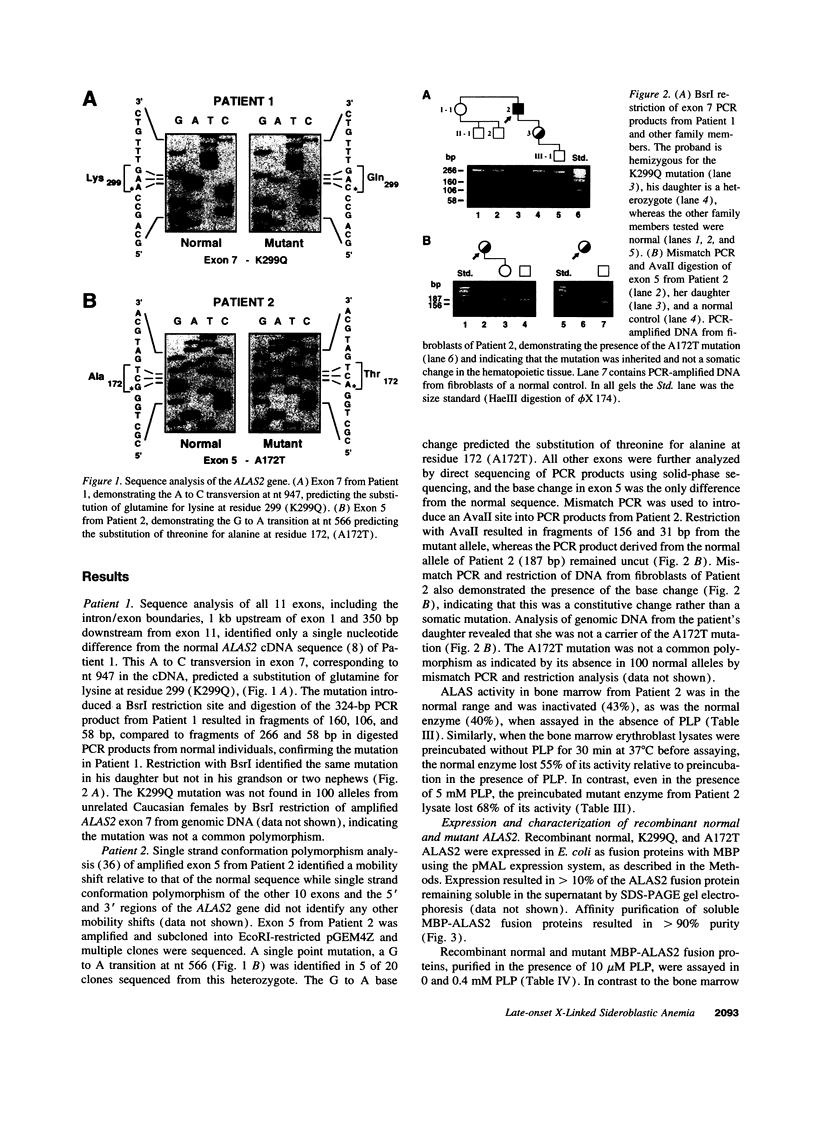
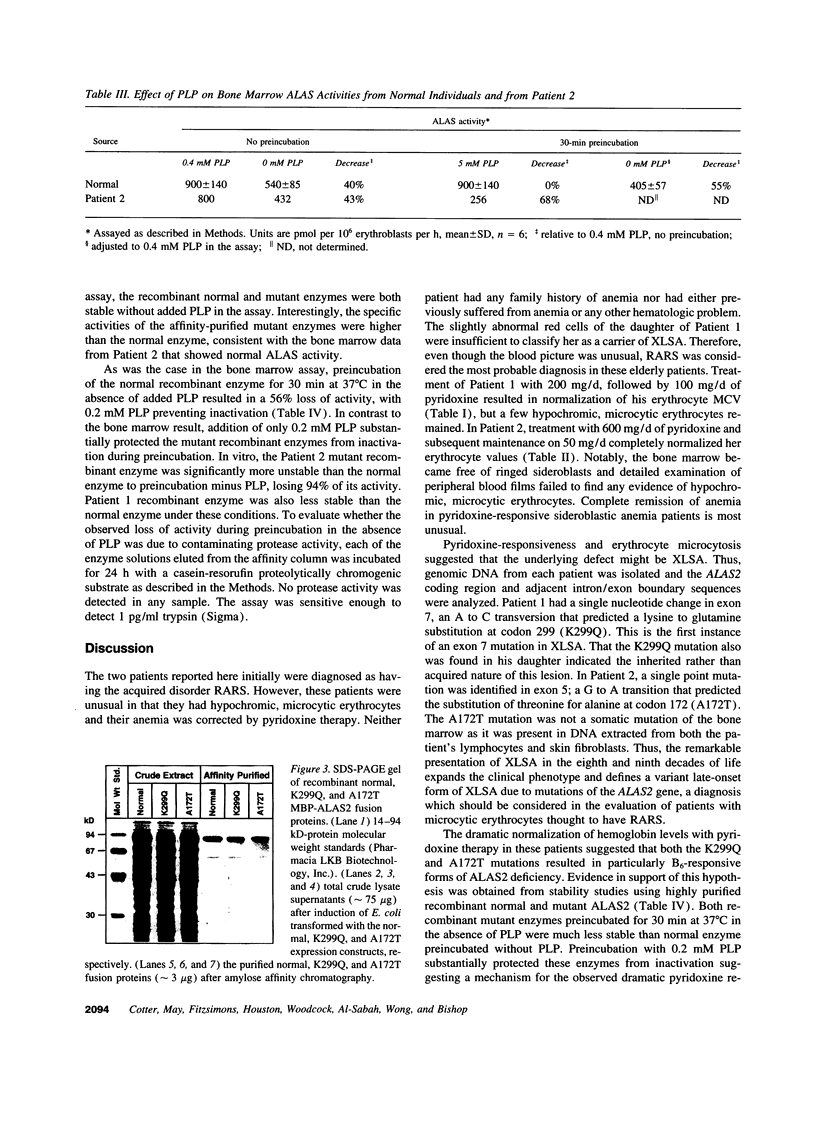
X-linked sideroblastic anemia (XLSA) is caused by mutations of the erythroid-specific delta-aminolevulinate synthase gene (ALAS2) resulting in deficient heme synthesis. The characteristic hypochromic, microcytic anemia typically becomes manifest in the first three decades of life. Hematologic response to pyridoxine is variable and rarely complete. We report two unrelated cases of highly pyridoxine-responsive XLSA in geriatric patients previously diagnosed with refractory anemia and ringed sideroblasts. A previously unaffected 77-yr-old male and an 81-yr-old female were each found to have developed severe hypochromic, microcytic anemia with ringed sideroblasts in the bone marrow, which responded dramatically to pyridoxine with normalization of hemoglobin values. Sequence analysis identified an A to C transversion in exon 7 (K299Q) of the ALAS2 gene in the male proband and his daughter. In the female proband a G to A transition was identified in exon 5 (A172T). This mutation resulted in decreased in vitro stability of bone marrow delta-aminolevulinate synthase activity. Each patient's recombinant mutant ALAS2 enzyme had marked thermolability. Addition of pyridoxal 5'-phosphate in vitro stabilized the mutant enzymes, consistent with the observed dramatic response to pyridoxine in vivo. This late-onset form of XLSA can be distinguished from refractory anemia and ringed sideroblasts by microcytosis, pyridoxine-responsiveness, and ALAS2 mutations. These findings emphasize the need to consider all elderly patients with microcytic sideroblastic anemia as candidates for XLSA, especially if pyridoxine responsiveness is demonstrated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki Y., Muranaka S., Nakabayashi K., Ueda Y. delta-Aminolevulinic acid synthetase in erythroblasts of patients with pyridoxine-responsive anemia. Hypercatabolism caused by the increased susceptibility to the controlling protease. J Clin Invest. 1979 Nov;64(5):1196–1203. doi: 10.1172/JCI109573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie D. S., Scotto A. W., Reddy U., DeLoskey R., Bosch C. G. Pyridoxal phosphate protects against an irreversible temperature-dependent inactivation of hepatic delta-aminolevulinic acid synthase. Arch Biochem Biophys. 1985 Jan;236(1):311–320. doi: 10.1016/0003-9861(85)90631-9. [DOI] [PubMed] [Google Scholar]
- Bennett J. M. Classification of the myelodysplastic syndromes. Clin Haematol. 1986 Nov;15(4):909–923. [PubMed] [Google Scholar]
- Bhasker C. R., Burgiel G., Neupert B., Emery-Goodman A., Kühn L. C., May B. K. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J Biol Chem. 1993 Jun 15;268(17):12699–12705. [PubMed] [Google Scholar]
- Bishop D. F. Two different genes encode delta-aminolevulinate synthase in humans: nucleotide sequences of cDNAs for the housekeeping and erythroid genes. Nucleic Acids Res. 1990 Dec 11;18(23):7187–7188. doi: 10.1093/nar/18.23.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Wampler D. E., Sgouris J. T., Bonefeld R. J., Anderson D. K., Hawley M. C., Sweeley C. C. Pilot scale purification of alpha-galactosidase A from Cohn fraction IV-1 of human plasma. Biochim Biophys Acta. 1978 May 11;524(1):109–120. doi: 10.1016/0005-2744(78)90109-2. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Wood W. A. An assay for delta-aminolevulinic acid synthetase based on a specific, semiautomatic determination of picomole quantities of delta-[14C]aminolevulinate. Anal Biochem. 1977 Jun;80(2):466–482. doi: 10.1016/0003-2697(77)90669-8. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Healy H. M., Brandenburg M. A., May B. K. 5-Aminolevulinate synthase in sideroblastic anemias: mRNA and enzyme activity levels in bone marrow cells. Am J Hematol. 1992 Oct;41(2):76–83. doi: 10.1002/ajh.2830410203. [DOI] [PubMed] [Google Scholar]
- Castañeda V. L., Williams T. E., Harper J. L., Graham-Pole J., Parmley R. T. Severe refractory anemia with ringed sideroblasts and bone marrow aplasia in a child. Am J Pediatr Hematol Oncol. 1992 Spring;14(1):70–76. doi: 10.1097/00043426-199221000-00011. [DOI] [PubMed] [Google Scholar]
- Cheng D. S., Kushner J. P., Wintrobe M. M. Idiopathic refractory sideroblastic anemia: incidence and risk factors for leukemic transformation. Cancer. 1979 Aug;44(2):724–731. doi: 10.1002/1097-0142(197908)44:2<724::aid-cncr2820440245>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Cotter P. D., Baumann M., Bishop D. F. Enzymatic defect in "X-linked" sideroblastic anemia: molecular evidence for erythroid delta-aminolevulinate synthase deficiency. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4028–4032. doi: 10.1073/pnas.89.9.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. D., Rucknagel D. L., Bishop D. F. X-linked sideroblastic anemia: identification of the mutation in the erythroid-specific delta-aminolevulinate synthase gene (ALAS2) in the original family described by Cooley. Blood. 1994 Dec 1;84(11):3915–3924. [PubMed] [Google Scholar]
- Cox T. C., Bottomley S. S., Wiley J. S., Bawden M. J., Matthews C. S., May B. K. X-linked pyridoxine-responsive sideroblastic anemia due to a Thr388-to-Ser substitution in erythroid 5-aminolevulinate synthase. N Engl J Med. 1994 Mar 10;330(10):675–679. doi: 10.1056/NEJM199403103301004. [DOI] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Fey M. F., Liechti-Gallati S., von Rohr A., Borisch B., Theilkäs L., Schneider V., Oestreicher M., Nagel S., Ziemiecki A., Tobler A. Clonality and X-inactivation patterns in hematopoietic cell populations detected by the highly informative M27 beta DNA probe. Blood. 1994 Feb 15;83(4):931–938. [PubMed] [Google Scholar]
- Fitzsimons E. J., May A., Elder G. H., Jacobs A. Measurement of 5-aminolevulinic acid synthase activity in whole and fractionated human bone marrow: effect of myeloid cell lysis by monoclonal antibody. Anal Biochem. 1986 Feb 15;153(1):9–17. doi: 10.1016/0003-2697(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Garand R., Gardais J., Bizet M., Bremond J. L., Accard F., Callat M. P., de Bouchony E. T., Goasguen J. E. Heterogeneity of acquired idiopathic sideroblastic anaemia (AISA). Leuk Res. 1992;16(5):463–468. doi: 10.1016/0145-2126(92)90171-3. [DOI] [PubMed] [Google Scholar]
- Gattermann N., Aul C., Schneider W. Two types of acquired idiopathic sideroblastic anaemia (AISA) Br J Haematol. 1990 Jan;74(1):45–52. doi: 10.1111/j.1365-2141.1990.tb02536.x. [DOI] [PubMed] [Google Scholar]
- HARRIS J. W., HORRIGAN D. L. PYRIDOXINE-RESPONSIVE ANEMIA--PROTOTYPE AND VARIATIONS ON THE THEME. Vitam Horm. 1964;22:721–753. [PubMed] [Google Scholar]
- HORRIGAN D. L., HARRIS J. W. PYRIDOXINE-RESPONSIVE ANEMIA: ANALYSIS OF 62 CASES. Adv Intern Med. 1964;12:103–174. [PubMed] [Google Scholar]
- Hast R., Bernell P. Minimal diagnostic criteria for the myelodysplastic syndrome in clinical practice. Leuk Res. 1992;16(1):8–9. doi: 10.1016/0145-2126(92)90094-n. [DOI] [PubMed] [Google Scholar]
- Hast R. Sideroblasts in myelodysplasia: their nature and clinical significance. Scand J Haematol Suppl. 1986;45:53–55. doi: 10.1111/j.1600-0609.1986.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine P. E., Cotter P. D., Johnson S. A., Fitzsimons E. J., Tyfield L., Lunt P. W., Bishop D. F. Pyridoxine-refractory congenital sideroblastic anaemia with evidence for autosomal inheritance: exclusion of linkage to ALAS2 at Xp11.21 by polymorphism analysis. J Med Genet. 1994 Mar;31(3):213–218. doi: 10.1136/jmg.31.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten E., van den Berg A., Riezler R., Naurath H. J., Lindenbaum J., Stabler S. P., Allen R. H. Metabolic evidence that deficiencies of vitamin B-12 (cobalamin), folate, and vitamin B-6 occur commonly in elderly people. Am J Clin Nutr. 1993 Oct;58(4):468–476. doi: 10.1093/ajcn/58.4.468. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- MACGIBBON B. H., MOLLIN D. L. SIDEROBLASTIC ANAEMIA IN MAN: OBSERVATIONS ON SEVENTY CASES. Br J Haematol. 1965 Jan;11:59–69. doi: 10.1111/j.1365-2141.1965.tb00085.x. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Meier P. J., Fehr J., Meyer U. A. Pyridoxine-responsive primary acquired sideroblastic anaemia. In vitro and in vivo effects of vitamin B6 on decreased 5-aminolaevulinate synthase activity. Scand J Haematol. 1982 Nov;29(5):421–424. [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Pagon R. A., Bird T. D., Detter J. C., Pierce I. Hereditary sideroblastic anaemia and ataxia: an X linked recessive disorder. J Med Genet. 1985 Aug;22(4):267–273. doi: 10.1136/jmg.22.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind W. H., Wijsman E., Pagon R. A., Cox T. C., Bawden M. J., May B. K., Bird T. D. X-linked sideroblastic anemia and ataxia: linkage to phosphoglycerate kinase at Xq13. Am J Hum Genet. 1991 Feb;48(2):335–341. [PMC free article] [PubMed] [Google Scholar]
- Sanz G. F., Sanz M. A., Vallespí T., del Cañizo M. C. Two types of acquired idiopathic sideroblastic anaemia. Br J Haematol. 1990 Aug;75(4):633–634. doi: 10.1111/j.1365-2141.1990.tb07824.x. [DOI] [PubMed] [Google Scholar]
- Twining S. S. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem. 1984 Nov 15;143(1):30–34. doi: 10.1016/0003-2697(84)90553-0. [DOI] [PubMed] [Google Scholar]
- van Waveren Hogervorst G. D., van Roermund H. P., Snijders P. J. Hereditary sideroblastic anaemia and autosomal inheritance of erythrocyte dimorphism in a Dutch family. Eur J Haematol. 1987 May;38(5):405–409. doi: 10.1111/j.1600-0609.1987.tb01436.x. [DOI] [PubMed] [Google Scholar]
- van den Berg H., Bode W., Mocking J. A., Löwik M. R. Effect of aging on vitamin B6 status and metabolism. Ann N Y Acad Sci. 1990;585:96–105. doi: 10.1111/j.1749-6632.1990.tb28045.x. [DOI] [PubMed] [Google Scholar]