Abstract
Pea weevil (Bruchus pisorum L.) oviposition on pods of specific genetic lines of pea (Pisum sativum L.) stimulates cell division at the sites of egg attachment. As a result, tumor-like growths of undifferentiated cells (neoplasms) develop beneath the egg. These neoplasms impede larval entry into the pod. This unique form of induced resistance is conditioned by the Np allele and mediated by a recently discovered class of natural products that we have identified from both cowpea weevil (Callosobruchus maculatus F.) and pea weevil. These compounds, which we refer to as “bruchins,” are long-chain α,ω-diols, esterified at one or both oxygens with 3-hydroxypropanoic acid. Bruchins are potent plant regulators, with application of as little as 1 fmol (0.5 pg) causing neoplastic growth on pods of all of the pea lines tested. The bruchins are, to our knowledge, the first natural products discovered with the ability to induce neoplasm formation when applied to intact plants.
Cell division in plants ordinarily occurs in meristems, and the newly formed cells differentiate to form plant tissues and organs (1, 2). In contrast, plant neoplasms, most commonly represented by various galls (3–5), arise when cell division is stimulated in nonmeristematic areas (1, 2). We study an interesting phenomenon observed in lines of pea (Pisum sativum L.) that exhibit the neoplastic pod phenotype, which is conferred by the wild-type allele, Neoplastic pod (Np), often found in pea germplasm (6, 7). This phenotype, first noted over 30 years ago, is typified by the formation of large patches of callus tissue (neoplasms) on the surface of pods grown under greenhouse conditions. Greenhouse coverings filter out the UV wavelengths from sunlight that ordinarily inhibit neoplasm formation (7). Plants possessing the Np gene grown under unfiltered sunlight in the field and plants homozygous for np do not form neoplasms. The neoplastic pod trait remained a poorly understood botanical curiosity until recently when it was reported that the Np gene conferred responsiveness to oviposition by the pea weevil (Bruchus pisorum L.), an economically important insect pest of pea, with neoplastic growth at the sites of egg attachment (8–10). Herein, we show that this response is a previously unidentified form of induced resistance to an insect, and we identify the insect-derived chemical signal that stimulates cell division and the resultant neoplastic growth.
Materials and Methods
Plant Material and Growing Conditions.
Lines of pea (P. sativum) homozygous for either the Np or np allele were used for all studies. For the field study described below and most bioassays, the lines used were selected from an F4 heterozygote from a cross between C887-332 (Np/Np) and I3 (np/np) (10). Pods to be used for bioassay were obtained from plants grown in a greenhouse with set points of 16°C and 21°C. Natural sunlight was supplemented with light from high-pressure sodium lamps that were on from 0600 until 2200 and a bank of eight cool white fluorescent tubes (F96T12/CW/SS) that were on continuously. Such supplemental lighting reduced the incidence of spontaneous neoplasm formation relative to that ordinarily seen on greenhouse-grown plants (7). A field study to compare weevil infestation on the Np/Np line with that on the np/np line was conducted in 1997 with five randomized blocks with 30 plants of each line per block. Plants were grown in raised beds on the Oregon State University Campus where they were exposed to a natural population of pea weevil (B. pisorum), an insect common in this area (11).
Pea Pod Bioassays.
Pods in the late flat pod stage (12) were split along the suture, and the half pod was placed in a Petri dish on moist filter paper with the outside surface exposed (10). Chromatographic fractions, appropriately diluted, and synthetic compounds to be tested for neoplasm-inducing activity were applied to the pod as 1-μl drops in 50% (vol/vol) ethanol. The bioassays were conducted in a growth chamber with continuous fluorescent light (30–40 microeinstein m−2⋅s−1) and a temperature of 23°C. The neoplastic tissue formed in 1 week was removed with a scalpel and weighed.
Histology and Microscopy.
Tissue to be used for light microscopy was fixed in 2–2.5% (vol/vol) glutaraldehyde in 0.1 M phosphate buffer, dehydrated through an alcohol series, embedded in paraffin, sectioned at 10 μm, and mounted on slides. The deparaffinized sections were stained with a Lillie–Mayer-type hematoxylin-eosin procedure. Specimens prepared for scanning electron microscopy were fixed as described above, critical point dried, and coated with gold:palladium (60:40, wt/wt).
Insect Collecting and Rearing.
Adult pea weevils were collected from experimental plantings of peas in eastern Washington, frozen, and shipped on dry ice to Corvallis, OR. Before extraction, the male and female insects were separated (13). Sexually mature female pea weevils were also obtained by collecting adults as they emerged from infested seed, determining their sex, and placing the females individually into Petri dishes containing several detached pea flowers (to serve as a source of pea pollen). As soon as egg deposition was noted (7–10 days), the insects were frozen and stored at −20°C.
A culture of cowpea weevils (Callosobruchus maculatus F.) provided by W. E. Burkholder (Stored Products Insects Research, U.S. Department of Agriculture, Agricultural Research Service, Madison, WI) was increased and used to inoculate 24 20-liter containers, each holding 500 g of chickpeas (Cicer arietinum L.). Cultures were held in the dark at room temperature. Adult insects were collected (by sieving) twice per week, frozen, and stored at −80°C. Chickpeas were replenished as required to maintain the cultures.
The vetch weevil, Bruchus brachialis F., was collected from wild vetch, Vicia villosa Roth., in eastern Washington (10). The bruchids Stator limbatus Horn and Stator pruininus Horn were provided in seeds of cat claw acacia, Acacia greggii A., by C. W. Fox (University of Kentucky, Lexington, KY).
Extraction and Isolation of Neoplasm-Inducing Compounds.
The first successful isolation began with a total lipid extraction procedure (14) that yielded 9 g of a viscous yellow oil from 100 g of adult cowpea weevils (about 22,000 insects). Flash chromatography was conducted with 2 g of this material with a 5 × 15-cm column of Florisil (Sigma F-9127) and with conditions as described for separation of simple lipid classes (15). Fractions were obtained by stepwise elution with hexane; 5, 15, and 30% (vol/vol) diethyl ether in hexane; diethyl ether; 2% (vol/vol) acetic acid in diethyl ether; and methanol.
An active fraction, 0.28 g of yellow oil, eluted with diethyl ether and acetic acid in diethyl ether, was passed over a 1 × 30-cm low-pressure, reversed-phase liquid chromatography column [C-18, J. T. Baker 7025-00, gradient elution starting with 85% (vol/vol) methanol in water and ending with 100% (vol/vol) methanol]; 80 7.5-ml fractions were collected. Bioassay indicated that activity was present in fractions 53–67, which were pooled (0.159 g of yellow oil).
Analysis of 10 mg of the pooled active fraction indicated that it was comprised largely of free fatty acids that were themselves inactive but were difficult to separate from the active materials. Accordingly, the remaining sample was reacted with 2-bromoacetophenone (Sigma B3145) to form the phenacyl esters of the fatty acids (16), thereby changing their chromatographic behavior and facilitating their separation from the active materials.
On repeating the low-pressure chromatographic separation described above with the 2-bromoacetophenone-treated material, two active samples were obtained, one eluting in fractions 56–60 (2.2 mg) and the other in fractions 64–67 (55 mg). The 2.2-mg fraction (light yellow oil) was subjected to TLC on a silver nitrate-impregnated silica gel plate [Whatman K5F; layer thickness = 0.25 mm; plate soaked in 12.5% (wt/vol) AgNO3 and activated at 80°C). After development, the plate edges were removed, sprayed with H2SO4:methanol (1:1, vol/vol), and charred; 11 fractions were taken based on appearance of the plate edges. Fraction 7 (white solid; Rf = 0.38–0.42; 0.4 mg), which was the most active on the basis of weight, was used for structure determination.
A refined and scaled-up version of this procedure was used to prepare additional active compounds starting with a 1,000-g sample of cowpea weevils. A similar but much scaled-down procedure was used to prepare active materials from a 4.96-g sample of field-collected female pea weevils (≈500 insects). In this case, HPLC was used instead of low-pressure liquid chromatography. The final active fractions obtained from pea weevils contained too little material for accurate weighing but could be analyzed by GC-MS.
Instrumentation.
NMR spectra were obtained on a Bruker DRX 600 spectrometer (Billerica, MA). Spectra were obtained in deuterochloroform. GC-MS was carried out with a Finnigan-MAT (San Jose, CA) Incos-50 GC-MS with a short (15-m × 0.25-mm i.d.) fused silica capillary column (DB-5, J & W Scientific, Folsom, CA). Both electron ionization-MS (70 V; block source temperature = 150°C) and chemical ionization-MS (ammonia or deuteroammonia as ionization gas; reagent gas pressure = 0.5 torr, 1 torr = 133 Pa; block temperature = 60°C) were used.
Derivatizations and Degradation.
Hydrolyses were achieved with 8% (wt/vol) NaOH in 85% (vol/vol) methanol at 70°C for 30 min. After neutralization with 2 M HCl, solvent was evaporated, and the residue was dissolved in ethyl acetate and passed through a very small column of silica gel. The substances in the eluate were analyzed by GC-MS or were derivatized for further analysis. Trimethylsilyl ethers were prepared by brief treatment with N,O-bis[trimethylsilyl]trifluoroacetamide at 60°C. Exhaustive hydrogenation/hydrogenolysis of the hydrolysis product was carried out over LiAlH4/Pt/Al2O3 at 300°C (17). Ozonolyses were conducted by collecting ozone in methylene chloride at −78°C and then treating cold methylene chloride solutions of the substrates with a few microliters of the ozone solution. The reactions were held outside the cooling bath for a few minutes and then quenched with dimethylsulfide.
Synthesis.
Unsaturated α,ω-diols were synthesized by standard routes involving acetylene alkylations and semihydrogenations and/or Wittig condensations. The (3-hydroxypropyl) esters were initially prepared by oxidative desilylation of 3-(phenyldimethylsilyl)propanoates as described for 1, (Z)-9-docosene-1,22-diol, 1-(3-hydroxypropanoate)ester (bruchin A, see below); 9-decyn-1-ol was deprotonated with butyllithium (two equivalents) in tetrahydrofuran and alkylated with the tetrahydropyranyl ether of 12-bromododecanol. The product was semihydrogenated (Lindlar catalyst; cyclohexene as solvent), and the olefinic alcohol was esterified with the acid chloride obtained by treating 3-(phenyldimethylsilyl) propanoic acid (18) with oxalyl chloride. After removal of the tetrahydropyranyl group (methanol; toluenesulfonic acid), the resulting monoester was treated with fluoroboric acid etherate in dichloromethane (room temperature; 5 h). Flash chromatography on silica gel (increasing portions of ethyl acetate in hexanes) was used to separate (Z)-9-docosene-1,22-diol (from ester hydrolysis) from the desired mono 3-(fluorodimethylsilyl)propanoate; the latter was then stirred at room temperature in methanol-tetrahydrofuran solution containing sodium bicarbonate, potassium fluoride, and 30% (vol/vol) hydrogen peroxide. After work-up and flash chromatography [40% then 50% (vol/vol) ethyl acetate in hexanes], 1 (bruchin A) was obtained as a white solid (melting point 47–48°C; shrink 45°C), after crystallization from heptane. 1H NMR (CDCl3, 600 MHz) d 5.34 (2H, m, olefinic), 4.11 (2H, t, J = 4.4 Hz, CH2CO2R), 3.86 (2H, t, J = 3.9 Hz, -O2CCH2CH2OH), 3.63 (2H, t, J = 4.6 Hz, alkyl-CH2OH), 2.70 (2H, t J = 3.8 Hz, -O2CCH2CH2OH), 2.09 (4H, m, allylic), 1.78 (br. s., OH). Electron ionization-MS, m/z (in percentages): 412 [M]+ (≈0.4), 124 (14), 123 (16), 121 (14), 111 (11), 109 (28), 97 (25), 96 (59), 95 (56), 94 (21), 93 (12), 91 (58), 83 (34), 82 (74), 81 (69), 80 (36), 79 (18), 73 (83), 71 (11), 69 (51), 68 (36), 67 (67), 57 (19), 56 (14), 55 (100), 54 (31), 45 (13), 43 (42), 42 (14), 41 (58). 1-bis(Trimethylsilyl) ether, electron ionization-MS m/z (in percentages): 556 [M]+ (≈0.2), 541 (≈0.2), 235 (10), 219 (22), 163 (31), 149 (10), 147 (100), 109 (13), 105 (28), 103 (79), 97 (12), 96 (14), 95 (20), 91 (12), 83 (17), 82 (14), 81 (23), 75 (58), 73 (57), 69 (24), 67 (23), 55 (41), 43 (15), 41 (16).
Results
Np Provides Resistance to Pea Weevil.
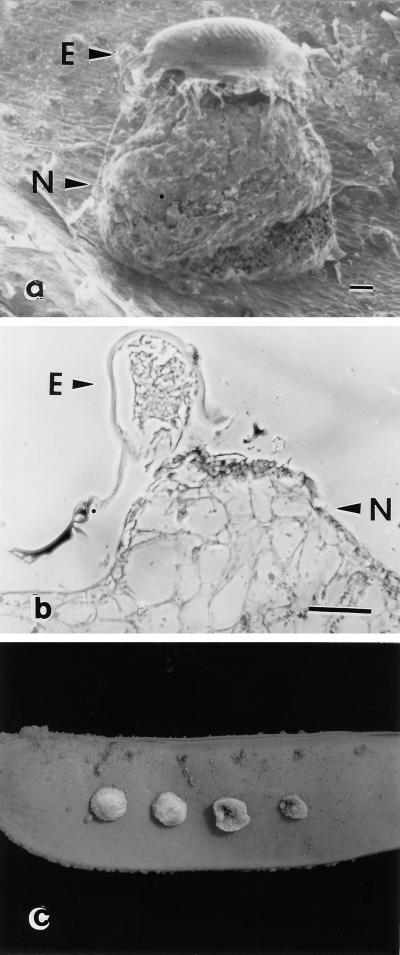
In a field trial with near-isogenic pea lines exposed to a natural population of pea weevil in Corvallis, OR, the rate of infestation of np/np seed was 85.4% versus 62.2% for Np/Np seed (P = 0.0017; blocks = 5). Neoplastic growth resulting from oviposition on Np/Np pods was clearly evident (Fig. 1 a and b).
Figure 1.
Stimulation of cell division on pods of an Np pea line (a derivative of C887-332; ref. 10) by several treatments. (a) Scanning electron micrograph showing the response of a pod from an Np pea line to oviposition by a pea weevil. The pod was obtained from a field-grown plant several days after oviposition. E, egg; N, neoplastic tissue formed in response to oviposition. (b) Cross section through a pod showing neoplastic tissue formed in response to pea weevil oviposition. Pod was harvested and fixed 8 days after oviposition. (c) Neoplasms present 1 week after application of various amounts of 2 (bruchin B; Fig. 4a), a compound present in extracts from the cowpea and pea weevil. Amounts applied as 1-μl drops in 50% (vol/vol) ethanol were (from left) 10, 5, 1, 0.5, and 0.0 pg. (Bars = 100 μm.)
Bruchids Contain Neoplasm-Inducing Activity.
Application of crude extracts, prepared with either freshly killed or frozen pea weevil adults, to Np pea pods resulted in neoplastic growth (Fig. 1c; ref. 10). Extracts of either pea weevil eggs or accompanying fluid were also mitogenic (10). The cowpea weevil, a bruchid that was reared more easily than the pea weevil, also yielded extracts that induced formation of neoplasms. In contrast to the pea weevil, where earlier work had demonstrated that sexually mature female insects were much richer sources of neoplasm-inducing activity than were males or immature females (10), newly emerged female or male cowpea weevils were equally good sources of activity. Consequently, this insect was used for initial isolation of neoplasm-inducing compounds. The sensitive and reliable Np pod assay (10) was used to guide fractionation of weevil extracts.
Characterization of Neoplasm-Inducing Compounds in Cowpea Weevil.
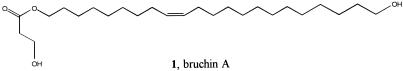
The mass spectrum of the first compound isolated (compound 1; Fig. 2) contained a barely detectable (0.4%) molecular ion. In many respects, it resembled the spectrum of oleyl alcohol with the addition of the prominent ions m/z 73 and 91. Chemical ionization with ammonia produced an ion with m/z 430, indicating a molecular weight of 412. Chemical ionization-MS with deuteroammonia demonstrated the presence of two exchangeable hydrogens, and formation of a bis-tetramethylsilane (bis-TMS) derivative on treatment with N,O-bis[trimethylsilyl]trifluoroacetamide was consistent with this conclusion.
Figure 2.
Bruchin A, a monoester bruchin isolated from a lipid extract of cowpea weevils.
On alkaline hydrolysis, 1 gave a new compound with molecular weight 340 whose mass spectrum lacked the ions at m/z 73 and 91. Exhaustive hydrogenation/hydrogenolysis (17) yielded n-docosane, n-heneicosane, and n-eicosane, indicating that the hydrolysis product was a docosene-1,22-diol. Indeed, catalytic hydrogenation (Pd/C; 1 atm; 1 atm = 101.3 kPa) provided docosane-1,22-diol. Ozonolysis gave rise to C9 and C13 hydroxyaldehydes, indicating that the diol possessed a 9-10 double bond.
The fact that the intact natural product contained two active hydrogens, one of which must have been present in the fragment lost during hydrolysis, suggested that the original compound could be a monoester of either lactic acid or the much less common 3-hydroxypropanoic acid. Published spectra of lactic acid esters did not contain the prominent m/z 73 and 91 ions, and spectra of esters of 3-hydroxypropanoic acid were not available. Accordingly, a small sample of the hydroxypropanoate of 1-decanol was prepared as a model compound. Its mass spectrum contained prominent m/z 73 and 91 ions, suggesting that the natural product was a mono 3-hydroxypropyl ester of the 9-docosene-1,22-diol.
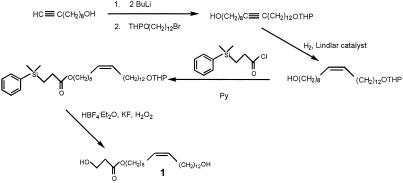
A 1-(3-hydroxypropanoate) ester with a (Z) double bond at the 9 position was prepared as illustrated in Fig. 3; a key element in the synthesis was the use of Fleming's masked hydroxy technology (19) for the construction of the 3-hydroxypropanoate group. We initially had no way of knowing which of the two OHs was esterified, nor did we have any information on the geometry of the double bond. We felt, however, that the natural product was of lipid origin and that the double-bond geometry would, accordingly, be (Z). (More detailed synthetic details appear in J.E.O., R.P.D., R.T.W., J.R.C., and E.D.D., unpublished work.) The synthetic 1 exhibited the same mass spectrum as the natural product, and GC retention times of the TMS derivatives were identical. Subsequent NMR experiments also confirmed the identity of the natural and synthetic compounds and supported the (Z) geometry assigned to the double bond. Synthetic 1 was as active in the pea pod bioassay as was the isolated compound.
Figure 3.
Synthesis scheme for (Z)-9-docosene-1,22-diol, 1-(3-hydroxypropanoate)ester (bruchin A), 1.
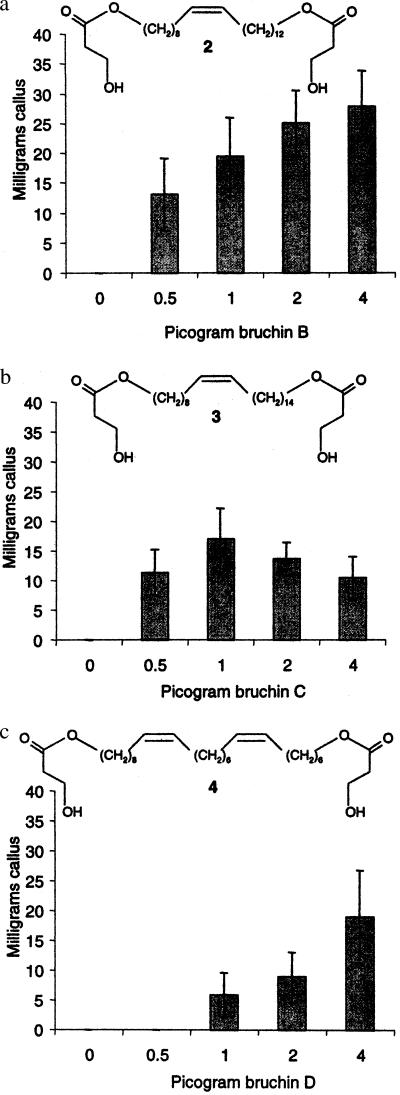
A second, somewhat refined, bioassay-guided fractionation of a 1,000-g sample of insects yielded three major active compounds (2–4, Fig. 4). All possessed the m/z 73 and 91 ions diagnostic for 3-(hydroxypropyl) esters. All formed bis-TMS ethers whose mass spectra contained ions with m/z of 103, 145, 147, and 163. [Although the first three of these ions are not uncommon in spectra of TMS ethers, particularly of polyfunctional compounds (20), collectively the four proved useful for selected ion monitoring-based identifications of TMS derivatives of both mono- and bis-3-hydroxypropyl esters.] Molecular weights from chemical ionization-MS were 628, 656, and 654. Hydrolyses were conducted as described above, and the molecular weight 628 compound (2) provided the same C22 diol characterized earlier. Hydrolysis of 3 and 4 gave monosaturated and diunsaturated C24 diols, respectively. These data, collectively, suggested that the active compounds were bis-3-(hydroxypropyl) diesters of α,ω-diols. Ozonolyses of the C24 diols demonstrated that the monounsaturated compound, as was the case with the C22 diol analyzed earlier, possessed a double bond at C9, whereas the diunsaturated diol had double bonds at C9 and C17. The C24 diols, each with (Z) double-bond geometry, were also synthesized with standard acetylene alkylations and/or Wittig condensations. Their NMR spectra confirmed the structural assignments, including the (Z) configurations for all double bonds. These three diesters were more abundant than the monoester characterized from the first isolation, and we believe that the bis-3-hydroxypropyl esters are the principal neoplasm-inducing agents. A more detailed discussion of the characterization and synthesis of 2–4 (bruchins B, C, and D, respectively) is included elsewhere (J.E.O., R.P.D., R.T.W., J.R.C., and E.D.D., unpublished work).
Figure 4.
Structures and activities of the principal bruchins present in sexually mature female pea weevils. Structures of bruchins B, C, and D are shown in a, b, and c, respectively. Neoplasms (calli) resulting from application of the indicated amounts of the bruchins were removed from pods with a scalpel and weighed 1 week after treatment (10). Each bar represents the mean ± SEM for six pods (blocks). Pea plants (derived from line C887-332; ref. 10) homozygous for the Np gene were grown in a greenhouse with light provided by a combination of high-pressure sodium lamps and fluorescent tubes. Bruchins were applied as 1-μl drops in 50% (vol/vol) ethanol to pods at the late flat pod stage (12).
Neoplasm-Inducing Compounds in Pea Weevil.
GC-MS analysis of fractionated whole-body extracts of adult female pea weevils identified the same three bis-3-(hydroxypropyl) esters (2–4) identified from the cowpea weevil. Monoester 1, if present, was far less abundant.
Neoplasm Formation in Response to Synthetic Compounds.
Synthetic versions of all three diesters (J.E.O., R.P.D., R.T.W., J.R.C., and E.D.D., unpublished work) were as active as the natural products in the pea pod bioassays (Fig. 4). As little as 1 fmol, ≈0.5 pg, when applied to an Np pea pod, resulted in neoplasms large enough to remove from the pods and weigh after 1 week.
Physiological Activity of Bruchins.
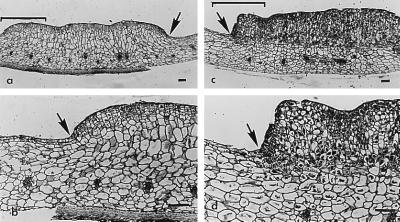
We propose the name, “bruchins” to refer to these neoplasm-inducing long-chain diols esterified at one or, more commonly, both oxygen atoms with 3-hydroxypropanoic acid. Bruchin application to Np pods causes browning of tissue at the treatment site within 3–6 h after initial exposure to the chemical. Swelling, resulting from mitosis in cells underlying the epidermis, is visible within 24–48 h (Fig. 5; ref. 10). Neoplasms, weighing from several hundred micrograms to several milligrams, are formed after 5–7 days (10). Bruchins do not cause callus formation when applied to leaves or stems of pea.
Figure 5.
Response of pea pods homozygous for either the Np or the np gene 96 h after application of 100 pg of 2, (bruchin B). The difference between np (a and b) and Np (c and d) pods, reflected as variation in the amount of cell division stimulated by the bruchin, is clearly visible. Arrows indicate margins of bruchin application, and brackets (a and c) indicate areas enlarged in b and d. Note the difference between the two phenotypes in the number of small, newly formed cells. Also note the influence of bruchin treatment on mesophyll cell size in treated and untreated regions of the np pods. The np pods were from a line, I3, derived from the common cultivar “Alaska.” All of the more than 20 np lines that we have tested show a similar response. The Np pods were from a line derived from a cross between I3 and line C887-332 (10). (Bars = 1 μm.)
Bruchin application also stimulates browning and swelling on pods homozygous for the np allele; however, in this case, much of the swelling results from cell enlargement rather than cell division, and neoplasms are usually too small to remove and weigh (Fig. 5). Pods of np/np plants typically fail to respond to pea weevil oviposition, although barely detectable swelling may occur under some eggs.
Discussion
It has been suggested, without direct evidence, that Np is a source of resistance to the pea weevil, a monophagous bruchid (8, 9, 21) that is one of the most serious insect pests of peas worldwide (22). Our use of isogenic lines confirmed such resistance even with large weevil populations. Neoplastic growth seems to provide resistance by reducing larval survival (8, 9, 21). Ordinarily, pea weevil larvae burrow through the ventral surface of the egg and directly into the pod to reach the immature seed (11). In contrast, eggs on Np pods are displaced from the pod surface by a mound of neoplastic tissue (Fig. 1 a and b). This mound causes the larvae to wander about before attempting to burrow through the pod wall, thus exposing them to environmental hazards including predators, parasites, and desiccation (9, 21). Moreover, the neoplasms with attached eggs are sometimes sloughed off of the pod before larval emergence (8).
We originally attempted to isolate neoplasm-inducing compounds from pea weevil but were unable to obtain sufficient numbers of this insect to provide enough active material for chemical characterization. Newly emerged female pea weevils must ingest fresh pollen to become sexually mature (10, 13), and only limited numbers of egg-bearing females could be collected from the field. Fortunately, the easily reared cowpea weevil provided a suitable alternative. Purified fractions were obtained by using this insect, and the distinctive mass spectrum of 3-(hydroxypropanoic) esters provided the key to identifying the bruchins.
Among the four bruchins described in this report, the monoester (Fig. 2) was the first identified, not because it was more abundant, but because it was the first active material obtained in a sufficiently pure state to allow characterization. In fact, subsequent isolations demonstrated that the diester bruchins (Fig. 4) were more abundant than the monoester.
Thus far, bruchins have been identified in adult insects of two bruchid genera. We have also detected strong neoplasm-inducing activity in crude extracts of the three other bruchid species that have been tested, namely: the vetch weevil, B. brachialis (10); S. limbatus; and S. pruininus. Moreover, we found that application of bruchin B (compound 2 of Fig. 4) to pods of Lathyrus tingitanus (Tangier peavine; PI 493288; “Raiano”) results in neoplasm formation. L. tingitanus is one of three legume species for which neoplastic growth in response to bruchid oviposition has been reported (8–10, 23). Similarly, oviposition on pods of certain lines of common bean, Phaseolus vulgaris L., by the bean-pod weevil, Apion godmani Wagner, a nonbruchid Coleopteran, results in callus formation that inhibits insect infestation (24). Hence, several legume species possess similar resistance mechanisms. However, bruchins have been identified only from pea weevil and cowpea weevil, and it is possible that unrelated compounds could induce the same response with peas as well as with other legumes.
It is noteworthy that pods of all pea lines tested, both Np and np, responded to bruchin application. The response of the np lines was attenuated, however. Only slight swelling occurred, even when large amounts of bruchin were applied (Fig. 5). We assume that the failure of np pods to react strongly to pea weevil oviposition is a result of this weak response and the low bruchin dose delivered with the egg and accompanying fluid.
We believe the bruchins to be the first regulators isolated from natural sources that stimulate neoplasm formation in intact plants. In peas possessing the Np allele, bruchins mediate a sensitive and efficient form of induced resistance wherein mitosis is stimulated and neoplasms form only in tissue in contact with the insect egg. Because bruchins are synthesized by the pea weevil at the expense of increased larval mortality, they must be “… the focus of intense selection pressure… ” (25). Their presence in the insect despite this pressure suggests that they play an important, but as yet undefined, role in the bruchid life cycle. There have been several reports of host-marking pheromones associated with the Bruchidae, including C. maculatus (26). However, preliminary tests indicate that bruchins do not play such a role in this insect.
The bruchins are relatively stable, low-melting, white solids, sparingly soluble in common solvents. Structurally, these long-chain α,ω-diols, monoesterified or diesterified with 3-hydroxypropanoic acid, represent a previously unknown class of compounds. In contrast to 3-hydroxybutanoic acid (27), its relatively important higher homolog, 3-hydroxypropanoic acid, was previously unknown as an element of natural products. The free acid is a hygroscopic liquid that is normally found only as a hydrate (28) and is mentioned only infrequently in the literature.
Preliminary results indicate that at least one 3-hydroxypropanoate functionality is required for activity. For example, the mono- and bis-(3-hydroxypropanoate) esters, bruchins A and B (1 and 2) have approximately equivalent activity, but the α,ω-diol itself is inactive. Although unsaturation in the diol chain is not required and the bis (3-hydroxypropanoate) ester of docosane-1,22-diol is fully active, the bis-propanoate, bis-lactate, and bis-(3-hydroxybutanoate) esters of this diol are virtually inactive. Further investigation will be necessary to identify the structural requirements for activity.
The ability of insects to manipulate lipids chemically for their own purposes is well established (29). It is noteworthy that the four bruchins characterized thus far resemble the common unsaturated fatty acids in possessing a (Z)-9 double bond. Despite the widely separated double bonds in bruchin D (4) that are in contrast to the usual 1,3 arrangement seen in polyunsaturated fatty acids, it seems likely that the bruchins are offshoots of fatty acid synthesis or metabolism. If so, the bruchins would join a slowly growing group of insect-produced difunctional fatty acid derivatives with unprecedented structures such as the defensive nitrogen-containing macrocycle from Epilachna varivestis recently reported by Attygalle et al. (30) and the remarkable volicitin from Pieris brassicae caterpillars. Volicitin, like the bruchins, initiates a complex plant-signaling sequence (31) that ultimately has a negative effect on the insect that produced the chemical.
Acknowledgments
We thank J. K. Christian and H. Throop for technical support and John Fellman for review of the manuscript. This work is technical paper 11642 of the Agricultural Experiment Station, Oregon State University.
Abbreviation
- TMS
tetramethylsilane
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110054697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110054697
References
- 1.Doonan J, Hunt T. Nature (London) 1996;380:481–482. doi: 10.1038/380481a0. [DOI] [PubMed] [Google Scholar]
- 2.Hemerly S S, Ferreira P C G, VanMontagu M, Inzé D. BioEssays. 1999;21:39–47. [Google Scholar]
- 3.Meyer J. Plant Galls and Gall Inducers. Berlin: Gebrüder Borntraeger; 1987. [Google Scholar]
- 4.Shorthouse J D, Rohrfritsch O E, editors. Biology of Insect-Induced Galls. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 5.Williams M J, editor. Plant Galls: Organisms, Interactions, Populations. Oxford: Clarendon; 1994. [Google Scholar]
- 6.Dodds K S, Matthews P. J Hered. 1966;57:83–85. [Google Scholar]
- 7.Snoad B, Matthews P. In: Chromosomes Today. Parlington C D, Lewis K R, editors. Edinburgh: Oliver Boyd; 1969. pp. 126–131. [Google Scholar]
- 8.Berdnikov V A, Trusov Y A, Bogdanova V S, Kosterin O E, Rozov S M, Nedel'kina S V, Nikulina Y N. Pisum Genet. 1992;24:37–39. [Google Scholar]
- 9.Hardie D. Ph.D. thesis. Adelaide, Australia: University of Adelaide; 1993. [Google Scholar]
- 10.Doss R P, Proebsting W M, Potter S W, Clement S L. J Chem Ecol. 1995;21:97–106. doi: 10.1007/BF02033665. [DOI] [PubMed] [Google Scholar]
- 11.Larson A O, Brindley T A, Hinman F G. Technical Bulletin. Washington, DC: U.S. Dept. Agric.; 1938. Publ. No. 599. [Google Scholar]
- 12.Meicenheimer R D, Muehlbauer F J. Exp Agric. 1982;18:17–27. [Google Scholar]
- 13.Pesho G R, VanHouten R J. Ann Entomol Soc Am. 1982;75:439–443. [Google Scholar]
- 14.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Christie W W. Lipid Analysis. Oxford: Pergamon Press; 1973. [Google Scholar]
- 16.Wood R, Lee T. J Chromatogr. 1983;254:237–246. [Google Scholar]
- 17.Bierl-Leonhardt B A, DeVilbiss E D. Anal Chem. 1981;53:936–938. [Google Scholar]
- 18.Takeshita K, Seik Y, Kawamoto K, Murai S, Sonoda N. J Org Chem. 1987;52:4864–4868. [Google Scholar]
- 19.Fleming J. Pure Appl Chem. 1988;60:71–78. [Google Scholar]
- 20.Halket J M. In: Handbook of Derivatives for Chromatography. Lau K, Halket J M, editors. New York: Wiley; 1993. pp. 297–325. [Google Scholar]
- 21.Clement S L, Cristofaro M, Cowgill S E, Weigand S. In: Global Plant Genetic Resources for Insect Resistant Crops. Clement S L, Quisenberry S S, editors. Boca Raton, FL: CRC; 1999. pp. 131–148. [Google Scholar]
- 22.Clement S L. Entomol Exp Appl. 1992;63:115–121. [Google Scholar]
- 23.Annis B, O'Keeffe L E. Entomol Exp Appl. 1984;35:83–87. [Google Scholar]
- 24.Garza R, Cardona C, Singh S P. Theor Appl Genet. 1996;92:357–362. doi: 10.1007/BF00223679. [DOI] [PubMed] [Google Scholar]
- 25.Farmer E E. Science. 1997;276:276–277. [Google Scholar]
- 26.Credmore P F, Wright A W. Physiol Entomol. 1990;15:285–298. [Google Scholar]
- 27.Müller H-M, Seeback D. Angew Chem Int Ed Engl. 1992;32:477–502. [Google Scholar]
- 28.Read D D. Organic Synthesis. I. New York: Wiley; 1964. pp. 321–324. [Google Scholar]
- 29.Stanley-Samuelson D W, Nelson D R, editors. Insect Lipids: Chemistry, Biochemistry, and Biology. Lincoln, NE: University of Nebraska Press; 1994. [Google Scholar]
- 30.Attygalle A B, Blankespoor C L, Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1994;91:12790–12793. doi: 10.1073/pnas.91.26.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paré P W, Alborn H T, Tumlinson J H. Proc Natl Acad Sci USA. 1998;95:13971–13975. doi: 10.1073/pnas.95.23.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]