Abstract
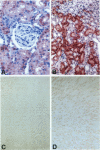
Macrophage migration inhibitory factor (MIF) is an important constituent of the host response to stress and infection and is the first mediator that has been identified to be released from immune cells upon stimulation with glucocorticoids. MIF also has been shown to be secreted from the anterior pituitary gland, monocytes/macrophages, and T cells activated by various proinflammatory stimuli. Once released, MIF acts to counter-regulate the inhibitory effect of glucocorticoids on inflammatory cytokine production. To characterize more precisely the role of MIF in the host response to infection, we undertook a systematic analysis of MIF expression in various organs of the rat after endotoxin (lipopolysaccharide) administration. MIF protein and mRNA were analyzed by immunohistochemistry and in situ hybridization, respectively. MIF was found to be expressed constitutively in organs such as the lung, liver, kidney, spleen, adrenal gland, and skin. Significant quantities of MIF protein were detected preformed in various cell types and appeared to be released as a consequence of endotoxemia. In virtually all tissues examined, the loss of MIF protein 6 hours after lipopolysaccharide administration was accompanied by the induction of MIF mRNA and, at 24 hours, by the restoration of immunoreactive, intracellular MIF. The constitutive production of MIF by several cell and tissue types together with its rapid release from intracellular pools distinguishes MIF from other cytokines or hormonal mediators and significantly expands the physiological role of this unique counter-regulator of glucocorticoid action.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardaillou R., Baud L. Production et activité proinflammatoire du facteur de nécrose tumorale alpha dans le glomérule. Bull Acad Natl Med. 1995 Jan;179(1):103–116. [PubMed] [Google Scholar]
- Bacher M., Metz C. N., Calandra T., Mayer K., Chesney J., Lohoff M., Gemsa D., Donnelly T., Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J., Bacher M., Calandra T., Metz C. N., Doty S. B., Donnelly T., Bucala R. An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996 Jan 1;183(1):277–282. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J., Calandra T., Mitchell R. A., Martin S. B., Tracey K. J., Voelter W., Manogue K. R., Cerami A., Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993 Oct 21;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Bernhagen J., Mitchell R. A., Calandra T., Voelter W., Cerami A., Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994 Nov 29;33(47):14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Bone R. C. The pathogenesis of sepsis. Ann Intern Med. 1991 Sep 15;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Bricio T., Molina A., Martin A., Mampaso F. In vitro modulation of interleukin-1 beta secretion by cultured rat doxorubicin-stimulated whole glomeruli and dissociated mesangial glomerular cells. Immunology. 1994 Jan;81(1):53–57. [PMC free article] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995 Sep 7;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Mitchell R. A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994 Jun 1;179(6):1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Remick D. G., Scales W. E., Kunkel S. L. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991 Feb;138(2):395–402. [PMC free article] [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg N., Freudenberg M. A., Bandara K., Galanos C. Distribution and localization of endotoxin in the reticulo-endothelial system (RES) and in the main vessels of the rat during shock. Pathol Res Pract. 1985 Mar;179(4-5):517–527. doi: 10.1016/S0344-0338(85)80193-X. [DOI] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Galat A., Rivière S., Bouet F. Purification of macrophage migration inhibitory factor (MIF) from bovine brain cytosol. FEBS Lett. 1993 Mar 22;319(3):233–236. doi: 10.1016/0014-5793(93)80553-7. [DOI] [PubMed] [Google Scholar]
- Ge Y., Ezzell R. M., Tompkins R. G., Warren H. S. Cellular distribution of endotoxin after injection of chemically purified lipopolysaccharide differs from that after injection of live bacteria. J Infect Dis. 1994 Jan;169(1):95–104. doi: 10.1093/infdis/169.1.95. [DOI] [PubMed] [Google Scholar]
- Kameda K., Sato K. Regulation of IL-1 alpha expression in human keratinocytes: transcriptional activation of the IL-1 alpha gene by TNF-alpha, LPS, and IL-1 alpha. Lymphokine Cytokine Res. 1994 Feb;13(1):29–35. [PubMed] [Google Scholar]
- Lan H. Y., Mu W., NG Y. Y., Nikolic-Paterson D. J., Atkins R. C. A simple, reliable, and sensitive method for nonradioactive in situ hybridization: use of microwave heating to improve hybridization efficiency and preserve tissue morphology. J Histochem Cytochem. 1996 Mar;44(3):281–287. doi: 10.1177/44.3.8648089. [DOI] [PubMed] [Google Scholar]
- Lan H. Y., Mu W., Yang N., Meinhardt A., Nikolic-Paterson D. J., Ng Y. Y., Bacher M., Atkins R. C., Bucala R. De Novo renal expression of macrophage migration inhibitory factor during the development of rat crescentic glomerulonephritis. Am J Pathol. 1996 Oct;149(4):1119–1127. [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Ulevitch R. J. Uptake and subcellular localization of bacterial lipopolysaccharide in the adrenal gland. Am J Pathol. 1985 Jul;120(1):79–86. [PMC free article] [PubMed] [Google Scholar]
- Mitchell R., Bacher M., Bernhagen J., Pushkarskaya T., Seldin M. F., Bucala R. Cloning and characterization of the gene for mouse macrophage migration inhibitory factor (MIF). J Immunol. 1995 Apr 15;154(8):3863–3870. [PubMed] [Google Scholar]
- Nishino T., Bernhagen J., Shiiki H., Calandra T., Dohi K., Bucala R. Localization of macrophage migration inhibitory factor (MIF) to secretory granules within the corticotrophic and thyrotrophic cells of the pituitary gland. Mol Med. 1995 Nov;1(7):781–788. [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Nishihira J., Hibiya Y., Koyama Y., Nishi S. Glutathione binding rat liver 13k protein is the homologue of the macrophage migration inhibitory factor. Biochem Mol Biol Int. 1994 Jun;33(3):439–446. [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Freeswick P. D., Khemlani L. S., Kispert P. H., Wang S. C., Su G. L., Billiar T. R. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun. 1995 Jul;63(7):2435–2442. doi: 10.1128/iai.63.7.2435-2442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Shaughnessy M. P., Lee D. C., Hodin J., Zelenka P. S. A macrophage migration inhibitory factor is expressed in the differentiating cells of the eye lens. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1272–1275. doi: 10.1073/pnas.90.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]