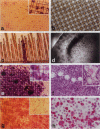
Abstract
We have developed a method to facilitate the isolation and expansion of tumor cells from body fluids and tissue biopsies. Antibody-conjugated magnetic beads (immunobeads) were used to isolate tumor cells from blood, bone marrow, ascitic/pleural fluids, and enzyme-digested tissue biopsies. Filtration of the resulting cell suspension through a 20-micron nylon monofilament filter secured to the base of polystyrene 96-well strips purged the bead-rosetting cell fraction of contaminating normal cells and unbound beads. Tumor cells that bound the magnetic beads were retained on the membrane due to their increased size and concentrated into a small area (0.332 cm2), thus maintaining a high cell density. The filters provided a stable and uniform three-dimensional matrix for cell growth, with a total surface area of 1.42 cm2 available for cell attachment. The filters could be easily removed from the base of the 96-well strips to facilitate handling and transfer between culture vessels. Tumor cells grown on the filters could subsequently be harvested using trypsin/EDTA or left in situ for immunostaining with conventional immunohistochemical procedures. Filter-grown cells have shown extended passage in conventional cell culture in six cases. In two of five cases, the orthotopic implantation of confluent filters that contained approximately 10(4) cells/8 x 8 mm filter successfully produced tumors in nude mice after only 4 weeks. Our new approach may be of value in improving the success rate of generating long-term cultures from previously unproductive sources of tumor cells and thus may yield a greater variety of cell lines/strains for the study of malignant disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker F. F., Wang X. B., Huang Y., Pethig R., Vykoukal J., Gascoyne P. R. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):860–864. doi: 10.1073/pnas.92.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Fidler I. J. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev. 1986;5(1):29–49. doi: 10.1007/BF00049529. [DOI] [PubMed] [Google Scholar]
- Fodstad O., Aamdal S., McMenamin M., Nesland J. M., Pihl A. A new experimental metastasis model in athymic nude mice, the human malignant melanoma LOX. Int J Cancer. 1988 Mar 15;41(3):442–449. doi: 10.1002/ijc.2910410322. [DOI] [PubMed] [Google Scholar]
- Fodstad O., Kjønniksen I., Aamdal S., Nesland J. M., Boyd M. R., Pihl A. Extrapulmonary, tissue-specific metastasis formation in nude mice injected with FEMX-I human melanoma cells. Cancer Res. 1988 Aug 1;48(15):4382–4388. [PubMed] [Google Scholar]
- Graham K. A., Trent J. M., Osborne C. K., McGrath C. M., Minden M. D., Buick R. N. The use of restriction fragment polymorphisms to identify the cell line MCF-7. Breast Cancer Res Treat. 1986;8(1):29–34. doi: 10.1007/BF01805922. [DOI] [PubMed] [Google Scholar]
- Kurebayashi J., Kurosumi M., Sonoo H. A new human breast cancer cell line, KPL-1 secretes tumour-associated antigens and grows rapidly in female athymic nude mice. Br J Cancer. 1995 Apr;71(4):845–853. doi: 10.1038/bjc.1995.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitz A. Development of tumor cell lines. Cancer Genet Cytogenet. 1986 Jan 1;19(1-2):11–19. doi: 10.1016/0165-4608(86)90366-3. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Scanlon E. F., Jelachich M. L., Klipstein S., Goldschmidt R. A. Growth and metastasis of human breast cancers in athymic nude mice. Clin Exp Metastasis. 1995 Jan;13(1):3–15. doi: 10.1007/BF00144013. [DOI] [PubMed] [Google Scholar]
- Pantel K., Dickmanns A., Zippelius A., Klein C., Shi J., Hoechtlen-Vollmar W., Schlimok G., Weckermann D., Oberneder R., Fanning E. Establishment of micrometastatic carcinoma cell lines: a novel source of tumor cell vaccines. J Natl Cancer Inst. 1995 Aug 2;87(15):1162–1168. doi: 10.1093/jnci/87.15.1162. [DOI] [PubMed] [Google Scholar]
- Parton R. G. Rapid processing of filter-grown cells for Epon embedding. J Histochem Cytochem. 1995 Jul;43(7):731–733. doi: 10.1177/43.7.7608529. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Alexander I. E., Koga M., Shine J., Sutherland R. L. Genetic instability and the development of steroid hormone insensitivity in cultured T 47D human breast cancer cells. Cancer Res. 1988 Aug 1;48(15):4340–4347. [PubMed] [Google Scholar]
- Ross A. A., Cooper B. W., Lazarus H. M., Mackay W., Moss T. J., Ciobanu N., Tallman M. S., Kennedy M. J., Davidson N. E., Sweet D. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood. 1993 Nov 1;82(9):2605–2610. [PubMed] [Google Scholar]
- Rubin H. Cellular epigenetics: effects of passage history on competence of cells for "spontaneous" transformation. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10715–10719. doi: 10.1073/pnas.90.22.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye P. D., Bovin N. V., Vlasova E., Høifødt H. K., Kierulf B., Trones G. E., Fodstad O. Breast cancer-associated antibody LU BCRU G7 recognises the terminal disaccharide Gal beta 1-3GlcNAc and can be used to isolate tumour cells from body fluids by an immunomagnetic bead procedure. Biochem Soc Trans. 1995 Nov;23(4):584S–584S. doi: 10.1042/bst023584s. [DOI] [PubMed] [Google Scholar]