Abstract
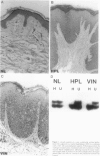
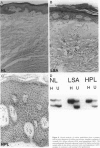
Squamous neoplasms of the female genital tract, including vulvar intraepithelial neoplasia, presumably are derived from a single cell. This study addressed this hypothesis and determined the clonal status of other squamous epithelial alterations associated with vulvar carcinoma, including hyperplasia and lichen sclerosis. X chromosome inactivation patterns of 22 epithelial lesions and matched normal epithelium were determined using a polymerase chain reaction (PCR)-based assay targeting the X-linked human androgen receptor gene (HUMARA). Clonality was inferred by comparing matched lesional and control tissues as follows: 1) monoclonal, if intensity of either PCR product was skewed relative to normal reference epithelium (control), 2) polyclonal, if both lesional and control were unskewed, and 3) unknown, if both lesion and control tissues were skewed toward the same allele. Two cases were excluded because of noninformative homozygous HUMARA alleles. Of 8 vulvar intraepithelial neoplasias analyzed, 7 were scored monoclonal and 1 polyclonal. Of 12 hyperplasias, 6 were monoclonal, including one with lichen sclerosis, 2 were polyclonal, and in 4, the clonal status could not be determined. The PCR-based clonal assay supports a monoclonal derivation for vulvar intraepithelial neoplasia and, in some cases, vulvar hyperplasia, and lichen sclerosis. The finding of monoclonal hyperplasia and lichen sclerosis suggests that clonal expansion may evolve before the development of morphological atypia in these epithelia.
Full text
PDF

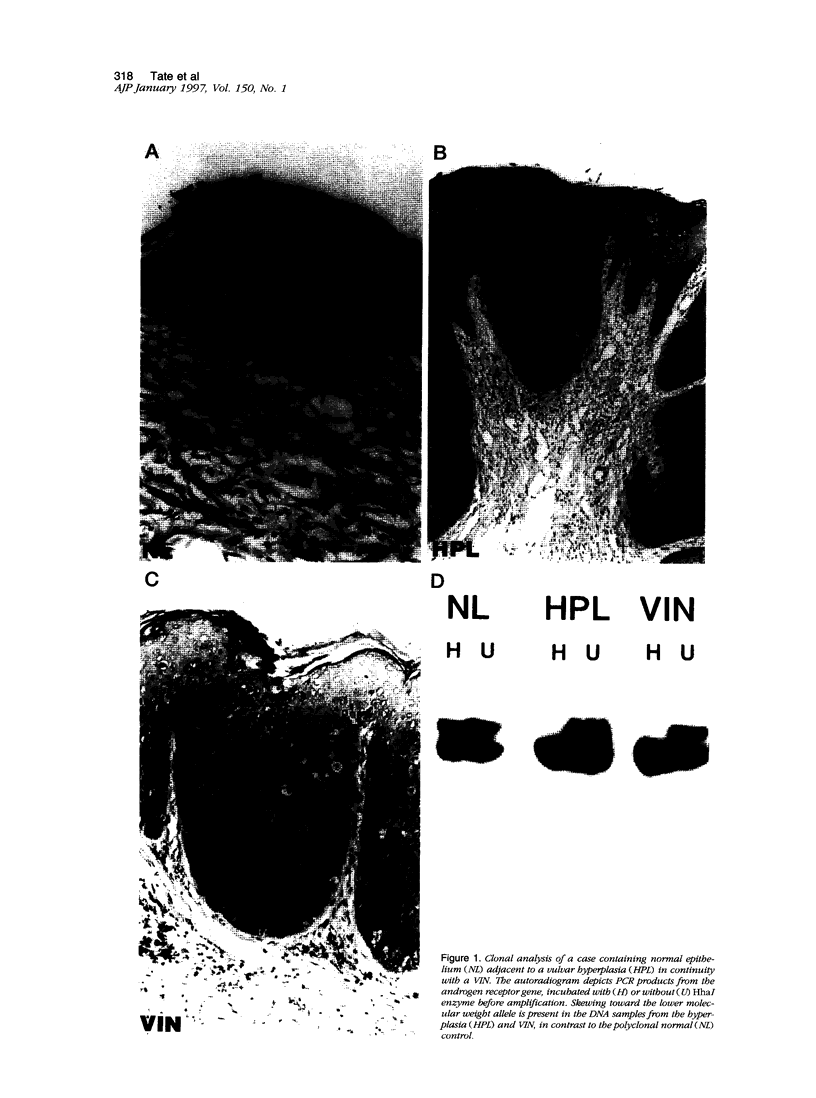
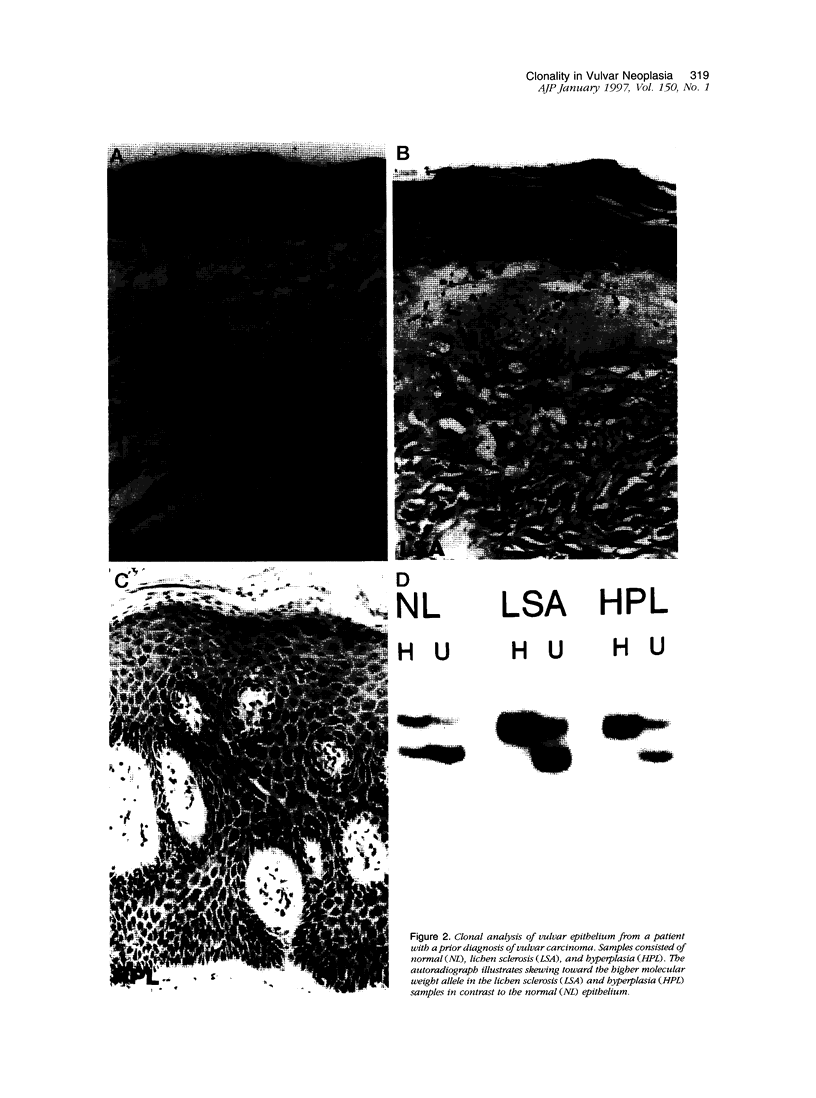



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Berg J. W., Lampe J. G. High-risk factors in gynecologic cancer. Cancer. 1981 Jul 15;48(2 Suppl):429–441. doi: 10.1002/1097-0142(19810715)48:1+<429::aid-cncr2820481303>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Brinton L. A., Nasca P. C., Mallin K., Baptiste M. S., Wilbanks G. D., Richart R. M. Case-control study of cancer of the vulva. Obstet Gynecol. 1990 May;75(5):859–866. [PubMed] [Google Scholar]
- Califano J., van der Riet P., Westra W., Nawroz H., Clayman G., Piantadosi S., Corio R., Lee D., Greenberg B., Koch W. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996 Jun 1;56(11):2488–2492. [PubMed] [Google Scholar]
- Cramer D. W., Cutler S. J. Incidence and histopathology of malignancies of the female genital organs in the United States. Am J Obstet Gynecol. 1974 Feb 15;118(4):443–460. doi: 10.1016/s0002-9378(16)33683-3. [DOI] [PubMed] [Google Scholar]
- Cramer D. W. Epidemiology of the gynecologic cancers. Compr Ther. 1978 Mar;4(3):9–17. [PubMed] [Google Scholar]
- Crum C. P. Carcinoma of the vulva: epidemiology and pathogenesis. Obstet Gynecol. 1992 Mar;79(3):448–454. doi: 10.1097/00006250-199203000-00025. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Primordial cell pool size and lineage relationships of five human cell types. Ann Hum Genet. 1973 Jul;37(1):39–48. doi: 10.1111/j.1469-1809.1973.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Fletcher J. A., Pinkus J. L., Lage J. M., Morton C. C., Pinkus G. S. Clonal 6p21 rearrangement is restricted to the mesenchymal component of an endometrial polyp. Genes Chromosomes Cancer. 1992 Oct;5(3):260–263. doi: 10.1002/gcc.2870050315. [DOI] [PubMed] [Google Scholar]
- GOSLING J. R., ABELL M. R., DROLETTE B. M., LOUGHRIN T. D. Infiltrative squamous cell (epidermoid) carcinoma of vulva. Cancer. 1961 Mar-Apr;14:330–343. doi: 10.1002/1097-0142(196103/04)14:2<330::aid-cncr2820140213>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Haefner H. K., Tate J. E., McLachlin C. M., Crum C. P. Vulvar intraepithelial neoplasia: age, morphological phenotype, papillomavirus DNA, and coexisting invasive carcinoma. Hum Pathol. 1995 Feb;26(2):147–154. doi: 10.1016/0046-8177(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Henson D., Tarone R. An epidemiologic study of cancer of the cervix, vagina, and vulva based on the Third National Cancer Survey in the United States. Am J Obstet Gynecol. 1977 Nov 1;129(5):525–532. [PubMed] [Google Scholar]
- Jovanovic A. S., Boynton K. A., Mutter G. L. Uteri of women with endometrial carcinoma contain a histopathological spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res. 1996 Apr 15;56(8):1917–1921. [PubMed] [Google Scholar]
- Krain L. S. Carcinoma of the vulva in California 1942-1969. The California Tumor Registry experience. Oncology. 1973;28(2):110–116. doi: 10.1159/000224808. [DOI] [PubMed] [Google Scholar]
- LINDER D., GARTLER S. M. DISTRIBUTION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE ELECTROPHORETIC VARIANTS IN DIFFERENT TISSUES OF HETEROZYGOTES. Am J Hum Genet. 1965 May;17:212–220. [PMC free article] [PubMed] [Google Scholar]
- Leibowitch M., Neill S., Pelisse M., Moyal-Baracco M. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990 Dec;97(12):1135–1139. doi: 10.1111/j.1471-0528.1990.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. Some milestones in the history of X-chromosome inactivation. Annu Rev Genet. 1992;26:16–28. doi: 10.1146/annurev.ge.26.120192.000313. [DOI] [PubMed] [Google Scholar]
- Menczer J., Voliovitch Y., Modan B., Modan M., Steinitz R. Some epidemiologic aspects of carcinoma of the vulva in Israel. Am J Obstet Gynecol. 1982 Aug 15;143(8):893–896. doi: 10.1016/0002-9378(82)90469-0. [DOI] [PubMed] [Google Scholar]
- Mutter G. L., Boynton K. A. PCR bias in amplification of androgen receptor alleles, a trinucleotide repeat marker used in clonality studies. Nucleic Acids Res. 1995 Apr 25;23(8):1411–1418. doi: 10.1093/nar/23.8.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I. J., Jones H. W., Jr Glucose-6-phosphate dehydrogenase and the histogenesis of epidermoid carcinoma of the cervix. Am J Obstet Gynecol. 1968 Sep 1;102(1):106–109. doi: 10.1016/0002-9378(68)90439-0. [DOI] [PubMed] [Google Scholar]
- Park T. W., Richart R. M., Sun X. W., Wright T. C., Jr Association between human papillomavirus type and clonal status of cervical squamous intraepithelial lesions. J Natl Cancer Inst. 1996 Mar 20;88(6):355–358. doi: 10.1093/jnci/88.6.355. [DOI] [PubMed] [Google Scholar]
- Peters R. K., Mack T. M., Bernstein L. Parallels in the epidemiology of selected anogenital carcinomas. J Natl Cancer Inst. 1984 Mar;72(3):609–615. [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Sengupta B. S. Carcinoma of the vulva in Jamaican women. Acta Obstet Gynecol Scand. 1981;60(6):537–544. doi: 10.3109/00016348109155482. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Townsend D. E., Sparkes R. S. Genetic variants of glucose-6-phosphate dehydrogenase in the study of carcinoma of the cervix. Cancer. 1971 Aug;28(2):529–532. doi: 10.1002/1097-0142(197108)28:2<529::aid-cncr2820280235>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Jonason A. S., Leffell D. J., Simon J. A., Sharma H. W., Kimmelman J., Remington L., Jacks T., Brash D. E. Sunburn and p53 in the onset of skin cancer. Nature. 1994 Dec 22;372(6508):773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]