Abstract
Malignant lymphomas frequently develop in the pleural cavity of patients with long-standing pyothorax. Thus, the term pyothorax-associated lymphoma (PAL) has been proposed for this type of tumor. Most PALs are diffuse lymphomas of B cell type and contain Epstein-Barr virus (EBV) DNA. We have established two lymphoma cell lines from the biopsy specimens of PAL cases, OPL-1 and OPL-2. Both cell lines contain EBV DNA, but only OPL-1 expresses EBV nuclear antigen 2, which works as a target molecule for the cell-mediated immune response. As systemic immunodeficiency is unlikely to be present in PAL patients, PAL from which OPL-1 derived was not expected to be fully developed. In this study, we examined the expression of immunosuppressive factors in OPLs. Only OPL-1, not OPL-2, expressed interleukin-10 (IL-10) mRNA and secreted IL-10 into culture supernatant. Both OPL-1 and OPL-2 expressed transforming growth factor (TGF)-beta 1 mRNA; however, neither expressed latent TGF-beta-binding protein mRNA at a detectable level by Northern blot analysis. Because TGF-beta expresses its functions in cooperation with latent TGF-beta-binding protein, the biological functions of TGF-beta 1 could be negligible. Neither cell line expressed at a detectable level EBV BCRF-1 mRNA, a viral gene product that is partly homologous to human IL-10 and shares biological activities of IL-10. Although IL-10 is reported to promote the growth of activated or neoplastic B cells, OPL-1 did not respond to human recombinant IL-10 by growing faster. As OPL-1 expresses a target antigen for the host cytotoxic T-cell response, the production of an immuno-suppressive cytokine, IL-10, might contribute to the development of overt lymphoma by inducing locally immunosuppressive circumstances. The present study suggests that an immunosuppressive cytokine plays a role in lymphomagenesis of immunocompetent patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baiocchi R. A., Caligiuri M. A. Low-dose interleukin 2 prevents the development of Epstein-Barr virus (EBV)-associated lymphoproliferative disease in scid/scid mice reconstituted i.p. with EBV-seropositive human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5577–5581. doi: 10.1073/pnas.91.12.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiocchi R. A., Ross M. E., Tan J. C., Chou C. C., Sullivan L., Haldar S., Monne M., Seiden M. V., Narula S. K., Sklar J. Lymphomagenesis in the SCID-hu mouse involves abundant production of human interleukin-10. Blood. 1995 Feb 15;85(4):1063–1074. [PubMed] [Google Scholar]
- Benjamin D., Knobloch T. J., Dayton M. A. Human B-cell interleukin-10: B-cell lines derived from patients with acquired immunodeficiency syndrome and Burkitt's lymphoma constitutively secrete large quantities of interleukin-10. Blood. 1992 Sep 1;80(5):1289–1298. [PubMed] [Google Scholar]
- Burdin N., Péronne C., Banchereau J., Rousset F. Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J Exp Med. 1993 Feb 1;177(2):295–304. doi: 10.1084/jem.177.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung R. K., Miyazaki I., Dosch H. M. Unexpected patterns of Epstein-Barr virus gene expression during early stages of B cell transformation. Int Immunol. 1993 Jul;5(7):707–716. doi: 10.1093/intimm/5.7.707. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft R., Abe M., Sato Y., Miyazono K., Harpel J., Heldin C. H., Rifkin D. B. Role of the latent TGF-beta binding protein in the activation of latent TGF-beta by co-cultures of endothelial and smooth muscle cells. J Cell Biol. 1993 Feb;120(4):995–1002. doi: 10.1083/jcb.120.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama M., Ibuka T., Hayashi Y., Ooba T., Koike M., Mizutani S. Epstein-Barr virus in pyothorax-associated pleural lymphoma. Am J Pathol. 1993 Oct;143(4):1044–1049. [PMC free article] [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Bankier A. T., Satchwell S. C., Barrell B. G. The short unique region of the B95-8 Epstein-Barr virus genome. Virology. 1985 Nov;147(1):81–98. doi: 10.1016/0042-6822(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Kanno H., Wolfinbarger J. B., Bloom M. E. Aleutian mink disease parvovirus infection of mink peritoneal macrophages and human macrophage cell lines. J Virol. 1993 Apr;67(4):2075–2082. doi: 10.1128/jvi.67.4.2075-2082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H., Yasunaga Y., Iuchi K., Yamauchi S., Tatekawa T., Sugiyama H., Aozasa K. Interleukin-6-mediated growth enhancement of cell lines derived from pyothorax-associated lymphoma. Lab Invest. 1996 Aug;75(2):167–173. [PubMed] [Google Scholar]
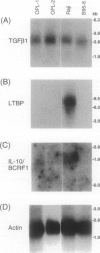
- Kanno H., Yasunaga Y., Ohsawa M., Taniwaki M., Iuchi K., Naka N., Torikai K., Shimoyama M., Aozasa K. Expression of Epstein-Barr virus latent infection genes and oncogenes in lymphoma cell lines derived from pyothorax-associated lymphoma. Int J Cancer. 1996 Jul 3;67(1):86–94. doi: 10.1002/(SICI)1097-0215(19960703)67:1<86::AID-IJC15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Kanzaki T., Olofsson A., Morén A., Wernstedt C., Hellman U., Miyazono K., Claesson-Welsh L., Heldin C. H. TGF-beta 1 binding protein: a component of the large latent complex of TGF-beta 1 with multiple repeat sequences. Cell. 1990 Jun 15;61(6):1051–1061. doi: 10.1016/0092-8674(90)90069-q. [DOI] [PubMed] [Google Scholar]
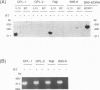
- Masood R., Zhang Y., Bond M. W., Scadden D. T., Moudgil T., Law R. E., Kaplan M. H., Jung B., Espina B. M., Lunardi-Iskandar Y. Interleukin-10 is an autocrine growth factor for acquired immunodeficiency syndrome-related B-cell lymphoma. Blood. 1995 Jun 15;85(12):3423–3430. [PubMed] [Google Scholar]
- Miyazaki I., Cheung R. K., Dosch H. M. Viral interleukin 10 is critical for the induction of B cell growth transformation by Epstein-Barr virus. J Exp Med. 1993 Aug 1;178(2):439–447. doi: 10.1084/jem.178.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K., Olofsson A., Colosetti P., Heldin C. H. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991 May;10(5):1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Vieira P., Fiorentino D. F., Trounstine M. L., Khan T. A., Mosmann T. R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990 Jun 8;248(4960):1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Brooks J. M., Thomas W. A., Rowe M., Kieff E., Rickinson A. B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992 Jul 1;176(1):157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa M., Tomita Y., Kanno H., Iuchi K., Kawabata Y., Nakajima Y., Komatsu H., Mukai K., Shimoyama M., Aozasa K. Role of Epstein-Barr virus in pleural lymphomagenesis. Mod Pathol. 1995 Oct;8(8):848–853. [PubMed] [Google Scholar]
- Ohshima K., Suzumiya J., Akamatu M., Takeshita M., Kikuchi M. Human and viral interleukin-10 in Hodgkin's disease, and its influence on CD4+ and CD8+ T lymphocytes. Int J Cancer. 1995 Jul 4;62(1):5–10. doi: 10.1002/ijc.2910620103. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986 Dec 26;47(6):883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Figari I. S., Espevik T., Palladino M. A., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickinson A. B., Murray R. J., Brooks J., Griffin H., Moss D. J., Masucci M. G. T cell recognition of Epstein-Barr virus associated lymphomas. Cancer Surv. 1992;13:53–80. [PubMed] [Google Scholar]
- Rooney C. M., Rowe M., Wallace L. E., Rickinson A. B. Epstein-Barr virus-positive Burkitt's lymphoma cells not recognized by virus-specific T-cell surveillance. Nature. 1985 Oct 17;317(6038):629–631. doi: 10.1038/317629a0. [DOI] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rowe D. T., Gregory C. D., Young L. S., Farrell P. J., Rupani H., Rickinson A. B. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 1987 Sep;6(9):2743–2751. doi: 10.1002/j.1460-2075.1987.tb02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Young L. S., Crocker J., Stokes H., Henderson S., Rickinson A. B. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991 Jan 1;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero D. A., Shoemaker R. H., Paull K. D., Monks A., Tierney S., Nofziger T. H., Currens M. J., Seniff D., Boyd M. R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988 Sep 1;48(17):4827–4833. [PubMed] [Google Scholar]
- Shibata D., Weiss L. M., Hernandez A. M., Nathwani B. N., Bernstein L., Levine A. M. Epstein-Barr virus-associated non-Hodgkin's lymphoma in patients infected with the human immunodeficiency virus. Blood. 1993 Apr 15;81(8):2102–2109. [PubMed] [Google Scholar]
- Tada T., Ohzeki S., Utsumi K., Takiuchi H., Muramatsu M., Li X. F., Shimizu J., Fujiwara H., Hamaoka T. Transforming growth factor-beta-induced inhibition of T cell function. Susceptibility difference in T cells of various phenotypes and functions and its relevance to immunosuppression in the tumor-bearing state. J Immunol. 1991 Feb 1;146(3):1077–1082. [PubMed] [Google Scholar]
- Thomas J. A., Hotchin N. A., Allday M. J., Amlot P., Rose M., Yacoub M., Crawford D. H. Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation. 1990 May;49(5):944–953. doi: 10.1097/00007890-199005000-00022. [DOI] [PubMed] [Google Scholar]
- Wolf H., Bogedain C., Schwarzmann F. Epstein-Barr virus and its interaction with the host. Intervirology. 1993;35(1-4):26–39. doi: 10.1159/000150293. [DOI] [PubMed] [Google Scholar]