Abstract
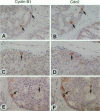
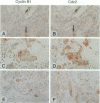
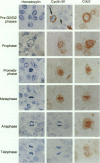
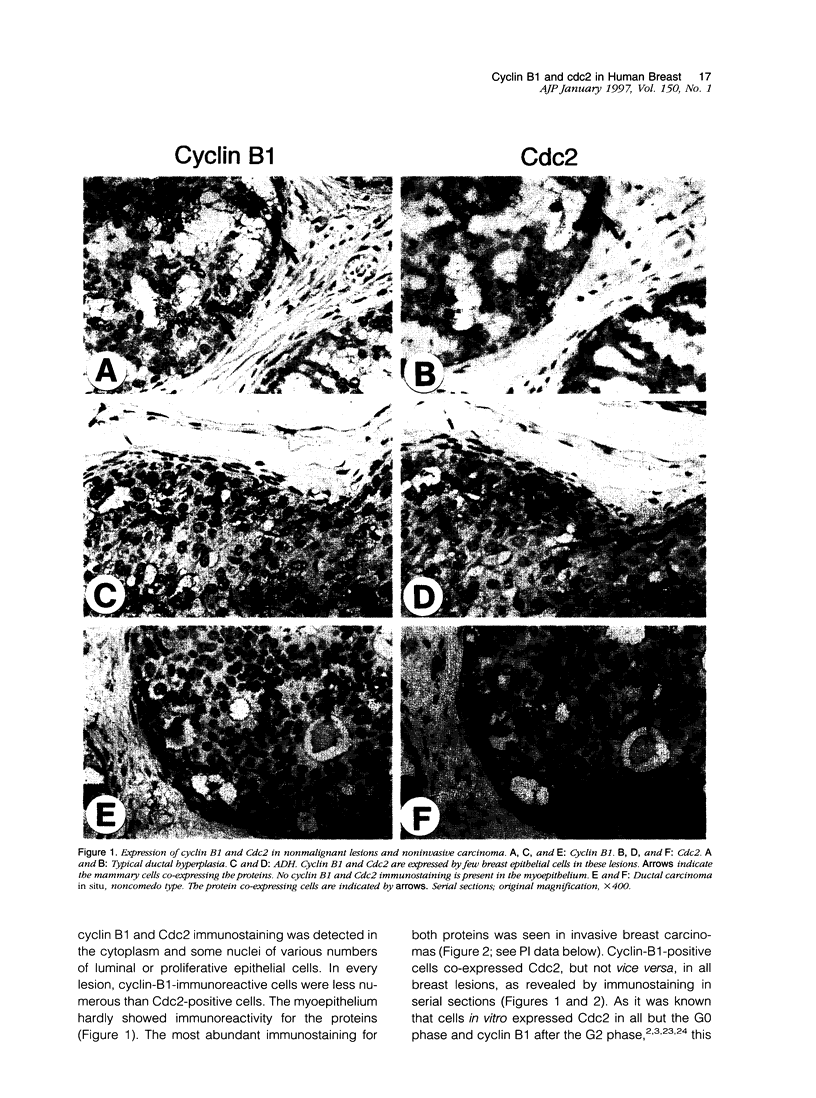
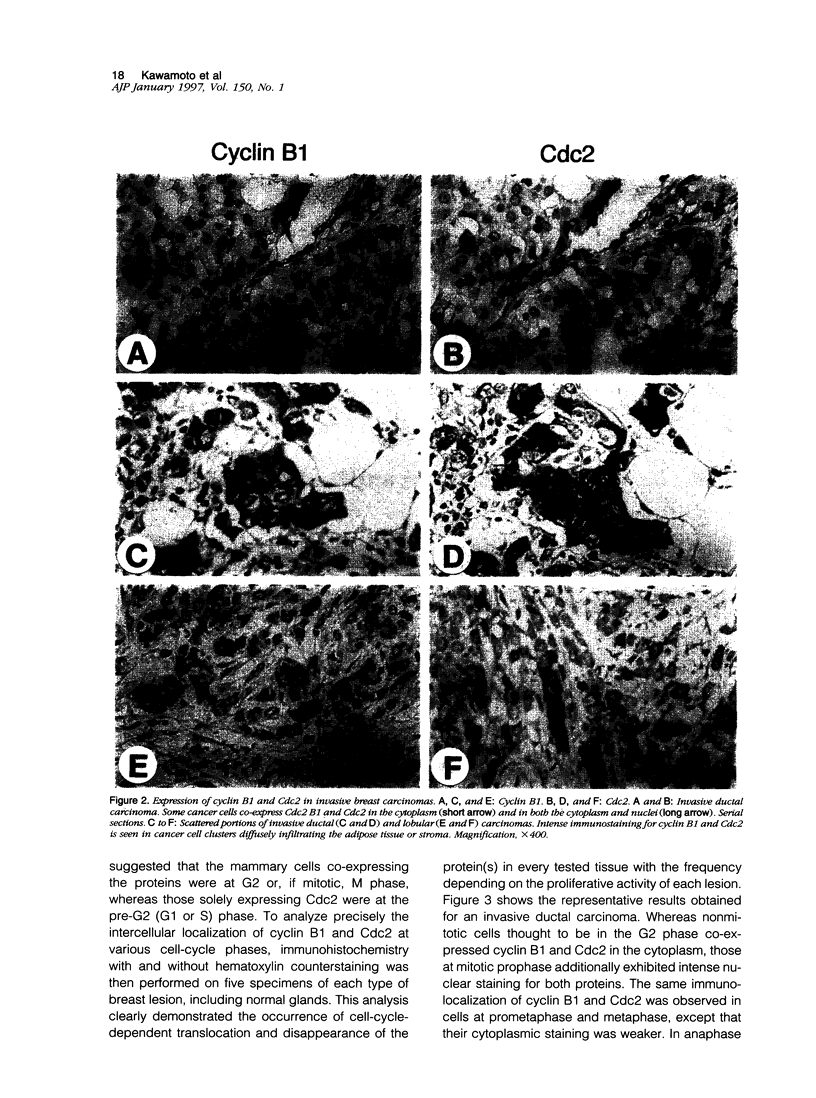
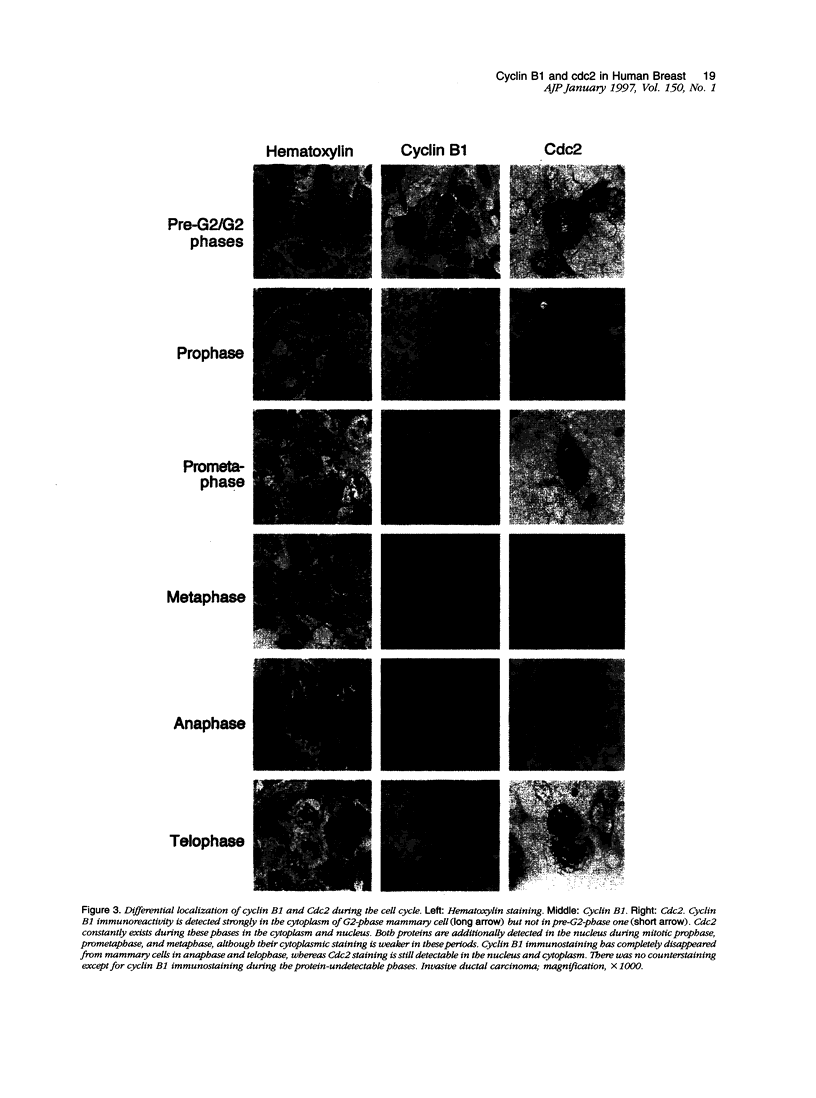
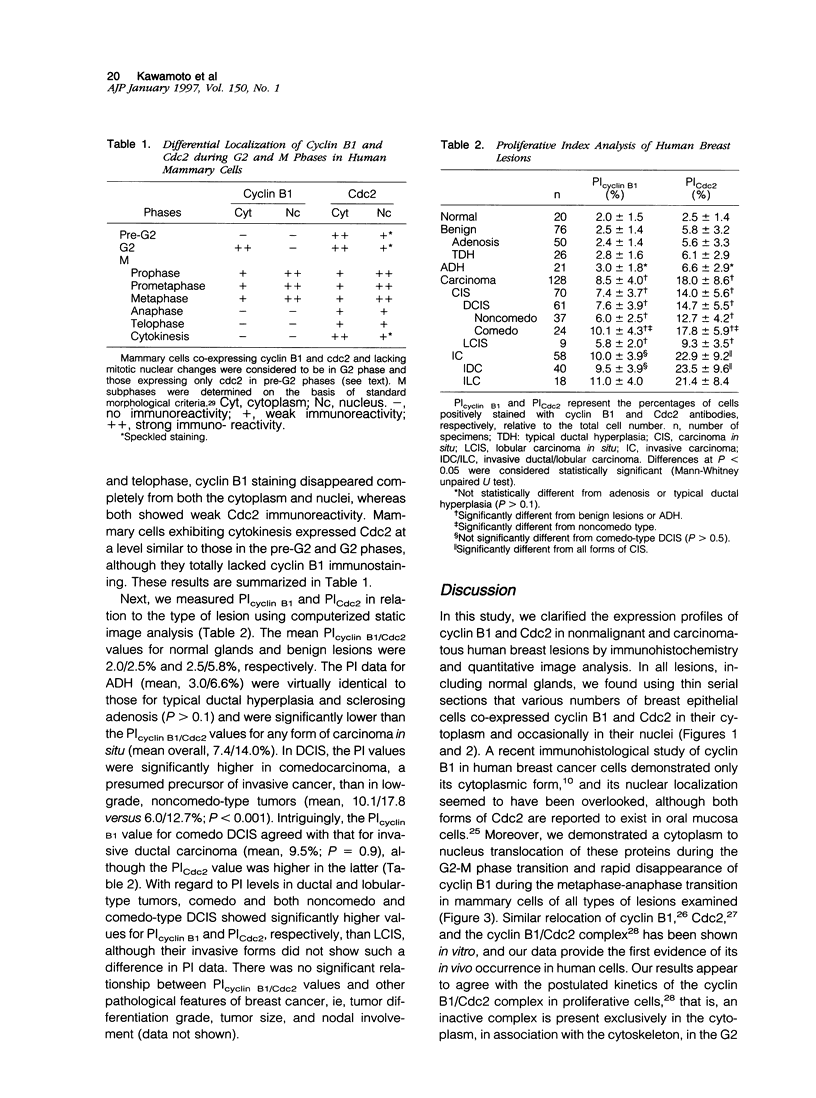
We investigated the in vivo expression of cyclin B1 and Cdc2 (key molecules for G2-M transition during the cell cycle) in nonmalignant and cancerous human breast lesions using immunohistochemistry and quantitative proliferative index (PI) analysis. Breast epithelial cells co-expressed cyclin B1 and Cdc2 in their cytoplasm in the G2 phase and in their nuclei in the M phase. Cyclin B1, but not Cdc2, immunostaining rapidly disappeared from the nuclei during the mitotic metaphase to anaphase transition. Static image analysis revealed the mean proliferative index for cyclin B1/cdc2 for each type of lesion to be as follows: normal glands (n = 20), 2.0/2.5%; benign lesions, including typical ductal hyperplasia (n = 76), 2.5/5.8%; atypical ductal hyperplasia (n = 21), 3.0/6.6%; carcinomas in situ (n = 70), 7.4/14.0%; and invasive carcinomas (n = 58), 10.0/22.9%. Proliferative index data for atypical hyperplasia were virtually identical to those for benign lesions and were significantly lower than those for breast cancer, suggesting that expression levels of cyclin B1 and Cdc2 may be used to distinguish premalignant human breast lesions from advanced disease. Furthermore, the proliferative index for cyclin B1 for comedo-type ductal carcinomas in situ agreed with that for invasive ductal carcinomas (mean, 10.1% versus 9.5%), apparently explaining the clinicopathological aggressiveness of this tumor at the molecular level.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afshari C. A., Barrett J. C. Disruption of G0-G1 arrest in quiescent and senescent cells treated with phosphatase inhibitors. Cancer Res. 1994 May 1;54(9):2317–2321. [PubMed] [Google Scholar]
- Bacus S. S., Goldschmidt R., Chin D., Moran G., Weinberg D., Bacus J. W. Biological grading of breast cancer using antibodies to proliferating cells and other markers. Am J Pathol. 1989 Nov;135(5):783–792. [PMC free article] [PubMed] [Google Scholar]
- Bailly E., Dorée M., Nurse P., Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989 Dec 20;8(13):3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley M. F., Sweeney K. J., Hamilton J. A., Sini R. L., Manning D. L., Nicholson R. I., deFazio A., Watts C. K., Musgrove E. A., Sutherland R. L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993 Aug;8(8):2127–2133. [PubMed] [Google Scholar]
- Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995 Sep;147(3):545–560. [PMC free article] [PubMed] [Google Scholar]
- Crissman H. A., Gadbois D. M., Tobey R. A., Bradbury E. M. Transformed mammalian cells are deficient in kinase-mediated control of progression through the G1 phase of the cell cycle. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7580–7584. doi: 10.1073/pnas.88.17.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont W. D., Page D. L. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985 Jan 17;312(3):146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- Dutta A., Chandra R., Leiter L. M., Lester S. Cyclins as markers of tumor proliferation: immunocytochemical studies in breast cancer. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5386–5390. doi: 10.1073/pnas.92.12.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett C., Fantl V., Smith R., Fisher C., Bartek J., Dickson C., Barnes D., Peters G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994 Apr 1;54(7):1812–1817. [PubMed] [Google Scholar]
- Goodger N. M., Gannon J., Hunt T., Morgan P. R. The localization of p34cdc2 in the cells of normal, hyperplastic, and malignant epithelial and lymphoid tissues of the oral cavity. J Pathol. 1996 Apr;178(4):422–428. doi: 10.1002/(SICI)1096-9896(199604)178:4<422::AID-PATH497>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Graña X., Reddy E. P. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene. 1995 Jul 20;11(2):211–219. [PubMed] [Google Scholar]
- Guillouf C., Rosselli F., Krishnaraju K., Moustacchi E., Hoffman B., Liebermann D. A. p53 involvement in control of G2 exit of the cell cycle: role in DNA damage-induced apoptosis. Oncogene. 1995 Jun 1;10(11):2263–2270. [PubMed] [Google Scholar]
- Hartwell L. H., Kastan M. B. Cell cycle control and cancer. Science. 1994 Dec 16;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Kerns B. J., Jordan P. A., Faerman L. L., Berchuck A., Bast R. C., Jr, Layfield L. J. Determination of proliferation index with MIB-1 in advanced ovarian cancer using quantitative image analysis. Am J Clin Pathol. 1994 Feb;101(2):192–197. doi: 10.1093/ajcp/101.2.192. [DOI] [PubMed] [Google Scholar]
- Keyomarsi K., Pardee A. B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Sukumar S., Barbacid M. Activation of ras oncogenes preceding the onset of neoplasia. Science. 1990 Jun 1;248(4959):1101–1104. doi: 10.1126/science.2188364. [DOI] [PubMed] [Google Scholar]
- Maller J. L. Mitotic control. Curr Opin Cell Biol. 1991 Apr;3(2):269–275. doi: 10.1016/0955-0674(91)90151-n. [DOI] [PubMed] [Google Scholar]
- McIntosh G. G., Anderson J. J., Milton I., Steward M., Parr A. H., Thomas M. D., Henry J. A., Angus B., Lennard T. W., Horne C. H. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene. 1995 Sep 7;11(5):885–891. [PubMed] [Google Scholar]
- McKinney C. D., Fechner R. E. Papillomas of the breast. A histologic spectrum including atypical hyperplasia and carcinoma in situ. Pathol Annu. 1995;30(Pt 2):137–178. [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nasmyth K., Hunt T. Cell cycle. Dams and sluices. Nature. 1993 Dec 16;366(6456):634–635. doi: 10.1038/366634a0. [DOI] [PubMed] [Google Scholar]
- Ohuchi N., Thor A., Page D. L., Hand P. H., Halter S. A., Schlom J. Expression of the 21,000 molecular weight ras protein in a spectrum of benign and malignant human mammary tissues. Cancer Res. 1986 May;46(5):2511–2519. [PubMed] [Google Scholar]
- Ookata K., Hisanaga S., Okano T., Tachibana K., Kishimoto T. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J. 1992 May;11(5):1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page D. L., Dupont W. D., Rogers L. W., Rados M. S. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985 Jun 1;55(11):2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Page D. L., Rogers L. W. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992 Oct;23(10):1095–1097. doi: 10.1016/0046-8177(92)90026-y. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I. The role of p34 kinases in the G1 to S-phase transition. Annu Rev Cell Biol. 1992;8:529–561. doi: 10.1146/annurev.cb.08.110192.002525. [DOI] [PubMed] [Google Scholar]
- Rosai J. Borderline epithelial lesions of the breast. Am J Surg Pathol. 1991 Mar;15(3):209–221. doi: 10.1097/00000478-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Rosen P. P. Proliferative breast "disease". An unresolved diagnostic dilemma. Cancer. 1993 Jun 15;71(12):3798–3807. doi: 10.1002/1097-0142(19930615)71:12<3798::aid-cncr2820711203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schnitt S. J., Connolly J. L., Tavassoli F. A., Fechner R. E., Kempson R. L., Gelman R., Page D. L. Interobserver reproducibility in the diagnosis of ductal proliferative breast lesions using standardized criteria. Am J Surg Pathol. 1992 Dec;16(12):1133–1143. doi: 10.1097/00000478-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Shibayama E., Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol. 1996 Jun;148(6):1807–1818. [PMC free article] [PubMed] [Google Scholar]
- Stein G. H., Drullinger L. F., Robetorye R. S., Pereira-Smith O. M., Smith J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstat-Saslow D., Merino M. J., Manrow R. E., Lawrence J. A., Bluth R. F., Wittenbel K. D., Simpson J. F., Page D. L., Steeg P. S. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med. 1995 Dec;1(12):1257–1260. doi: 10.1038/nm1295-1257. [DOI] [PubMed] [Google Scholar]
- Zhang S. Y., Caamano J., Cooper F., Guo X., Klein-Szanto A. J. Immunohistochemistry of cyclin D1 in human breast cancer. Am J Clin Pathol. 1994 Nov;102(5):695–698. doi: 10.1093/ajcp/102.5.695. [DOI] [PubMed] [Google Scholar]