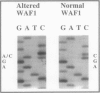
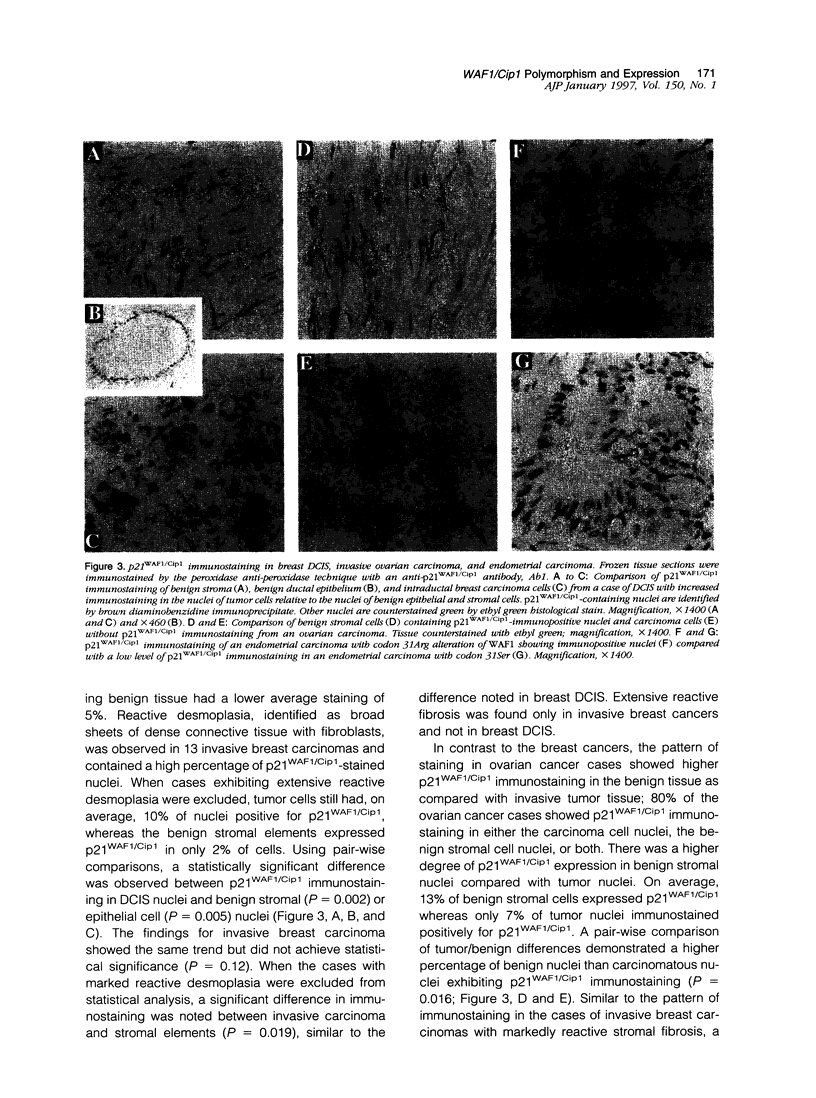
Abstract
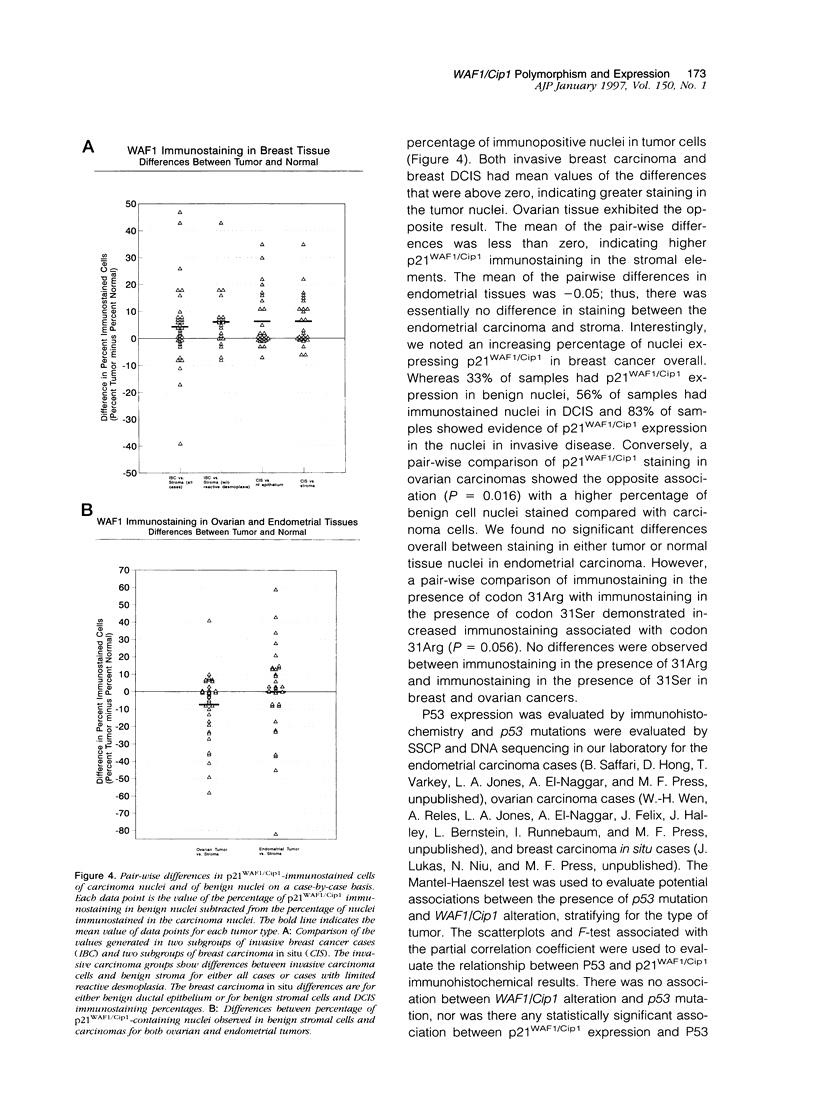
The p53 gene is altered in approximately 50% of human cancers and is considered to be important in the pathogenesis of these malignancies. The p53 protein product regulates the transition from G1 to S phase of the cell cycle and entry to the DNA damage repair pathway. As alterations in this pathway appear to be important in a variety of human cancers, downstream effector proteins of p53 are potential sites for somatic alterations. WAF1/Cip1, also known as WAF1, Cip1, sdi1, or CAP20, codes for a 21-kd protein (p21WAF1/Cip1), which was recently described as a universal inhibitor of cyclins and is thus critical in cell cycle control. Mutations in WAF1/Cip1 are potentially important in human malignancies because they could affect the control of the cell cycle. To understand whether mutations of WAF1/Cip1 occur in cancer, we screened 53 cases of invasive breast carcinoma, 35 cases of ductal carcinoma in situ (DCIS), 53 ovarian carcinomas, and 47 endometrial carcinomas in the second exon of WAF1/Cip1 (90% of the open reading frame). p21WAF1/Cip1 expression was characterized with immunohistochemistry. Cells from the blood of 21 normal individuals were also characterized using single-strand conformational polymorphism analysis, DNA sequencing and restriction analysis. Single-strand conformational polymorphism analysis demonstrated an altered mobility pattern for exon 2 in 12 invasive breast cancers (22.6%), 5 DCIS of the breast (14%), 8 invasive ovarian carcinomas (15%), and 9 endometrial carcinomas (19%). In total, 209 samples were screened, and 38 cases (18.2%) had an altered codon 31. Each case with altered single-strand conformational polymorphism, analyzed by DNA sequencing and/or restriction analysis, showed the same alteration of codon 31, a C to A transversion encoding a change in amino acid sequence from serine to arginine (31Ser-->31Arg). DNA from the blood of 21 normal individuals showed the same alteration in WAF1/Cip1 in 4 cases (19%). Furthermore, paired normal tissue was available for 3 of 20 breast carcinomas with the Ser31Arg transversion. Normal DNA from all 3 cases showed the same 31Arg alteration as found in the tumor tissue. These results indicate that codon 31 is a polymorphic site and that the serine to arginine shift is a polymorphism. p21WAF1/Cip1 expression, identified by immunohistochemistry, was found to vary in a pattern that depended both on the tissue type and on the presence or absence of the codon 31 polymorphism. Using pair-wise comparisons in breast DCIS, we found higher protein expression in tumor nuclei as compared with benign stromal cell nuclei (P = 0.002) or normal ductal epithelium (P = 0.005). Invasive breast cancer specimens showed a trend in p21WAF1/Cip1 immunostaining similar to DCIS but did not reach statistical significance (P = 0.12). However, when cases with extensive desmoplastic reaction were excluded, a statistically significant association (P = 0.019) similar to that in DCIS was noted. In contrast to the breast tumors, ovarian carcinomas exhibited significantly greater p21WAF1/Cip1 expression in the benign stromal (fibroblast) nuclei surrounding the tumor than in the carcinoma cell nuclei (P = 0.016). Endometrial carcinoma revealed no difference in staining when comparing benign tissue with carcinoma (P = 0.99); however, unlike breast and ovarian carcinomas in which there was no correlation between p21WAF1/Cip1 expression and the presence or absence of the alteration at the 31st codon, endometrial carcinomas showed an increased percentage of immunopositive nuclei associated (P = 0.056) with 31Arg. These results demonstrate tissue-specific expression patterns of WAF1/Cip1 in different tumors which appears to be characteristic of the tumor type.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chedid M., Michieli P., Lengel C., Huppi K., Givol D. A single nucleotide substitution at codon 31 (Ser/Arg) defines a polymorphism in a highly conserved region of the p53-inducible gene WAF1/CIP1. Oncogene. 1994 Oct;9(10):3021–3024. [PubMed] [Google Scholar]
- Datto M. B., Li Y., Panus J. F., Howe D. J., Xiong Y., Wang X. F. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Reynisdóttir I., Massagué J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995 Mar 15;9(6):639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- Li Y., Jenkins C. W., Nichols M. A., Xiong Y. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene. 1994 Aug;9(8):2261–2268. [PubMed] [Google Scholar]
- Matsuoka S., Edwards M. C., Bai C., Parker S., Zhang P., Baldini A., Harper J. W., Elledge S. J. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995 Mar 15;9(6):650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- Michaud J., Brody L. C., Steel G., Fontaine G., Martin L. S., Valle D., Mitchell G. Strand-separating conformational polymorphism analysis: efficacy of detection of point mutations in the human ornithine delta-aminotransferase gene. Genomics. 1992 Jun;13(2):389–394. doi: 10.1016/0888-7543(92)90258-t. [DOI] [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Nakanishi M., Robetorye R. S., Adami G. R., Pereira-Smith O. M., Smith J. R. Identification of the active region of the DNA synthesis inhibitory gene p21Sdi1/CIP1/WAF1. EMBO J. 1995 Feb 1;14(3):555–563. doi: 10.1002/j.1460-2075.1995.tb07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar-Stewart V., Motulsky A. G., Eaton D. L., White E., Hornung S. K., Leng Z. T., Stapleton P., Weiss N. S. The glutathione S-transferase mu polymorphism as a marker for susceptibility to lung carcinoma. Cancer Res. 1993 May 15;53(10 Suppl):2313–2318. [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Ponce-Castañeda M. V., Lee M. H., Latres E., Polyak K., Lacombe L., Montgomery K., Mathew S., Krauter K., Sheinfeld J., Massague J. p27Kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res. 1995 Mar 15;55(6):1211–1214. [PubMed] [Google Scholar]
- Press M. F., Nousek-Goebl N., King W. J., Herbst A. L., Greene G. L. Immunohistochemical assessment of estrogen receptor distribution in the human endometrium throughout the menstrual cycle. Lab Invest. 1984 Nov;51(5):495–503. [PubMed] [Google Scholar]
- Shiohara M., el-Deiry W. S., Wada M., Nakamaki T., Takeuchi S., Yang R., Chen D. L., Vogelstein B., Koeffler H. P. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994 Dec 1;84(11):3781–3784. [PubMed] [Google Scholar]
- Taioli E., Crofts F., Trachman J., Demopoulos R., Toniolo P., Garte S. J. A specific African-American CYP1A1 polymorphism is associated with adenocarcinoma of the lung. Cancer Res. 1995 Feb 1;55(3):472–473. [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Zhang H., Hannon G. J., Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994 Aug 1;8(15):1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- Zhang H., Xiong Y., Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993 Sep;4(9):897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995 Jul 1;55(13):2910–2919. [PubMed] [Google Scholar]