Abstract
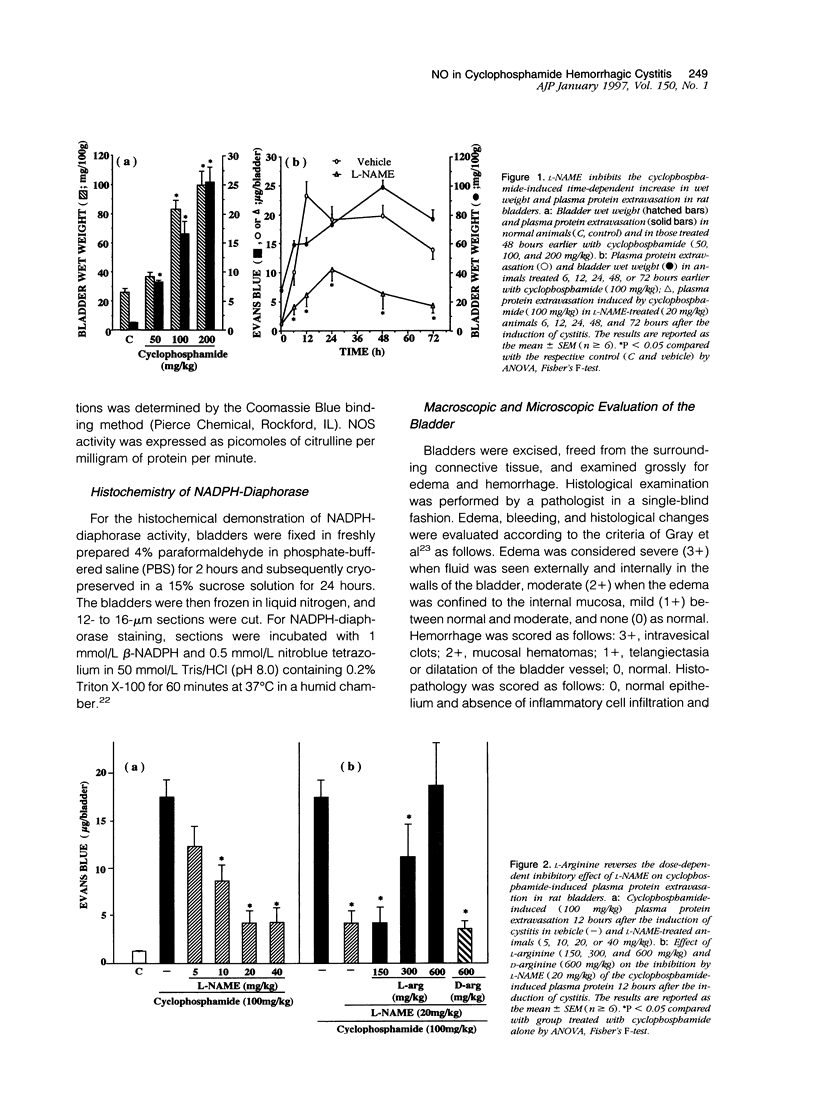
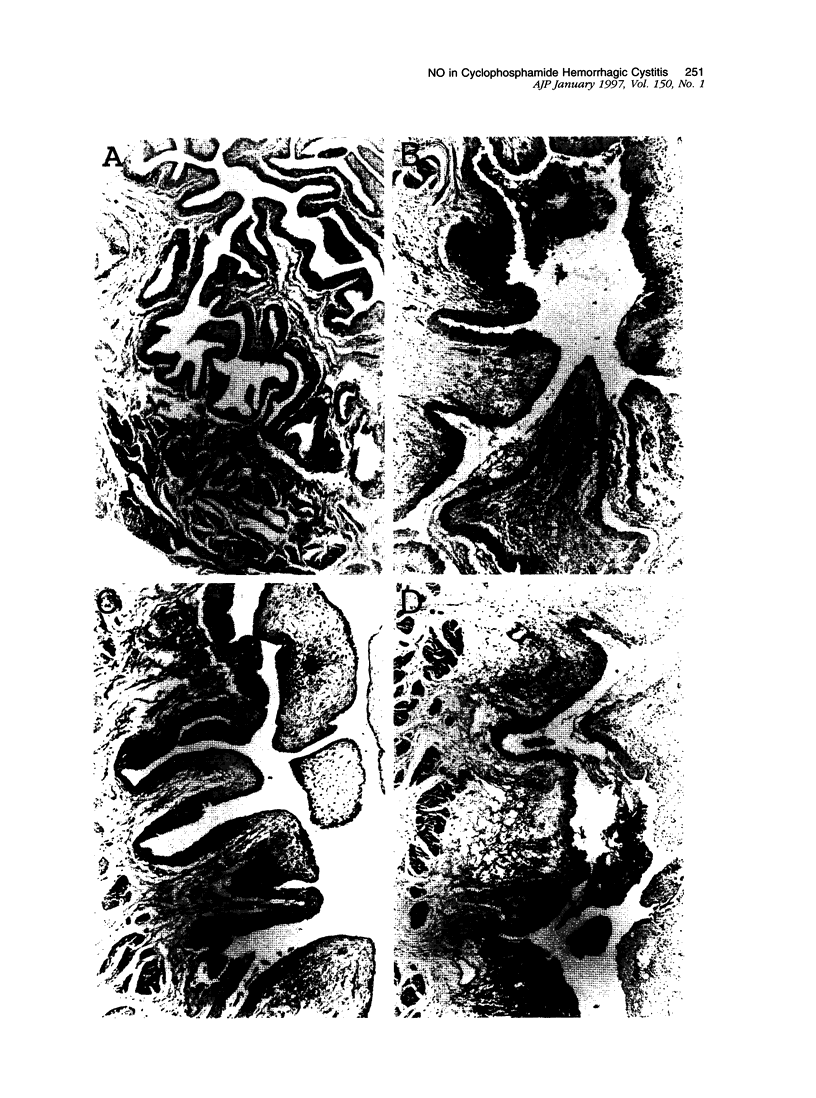
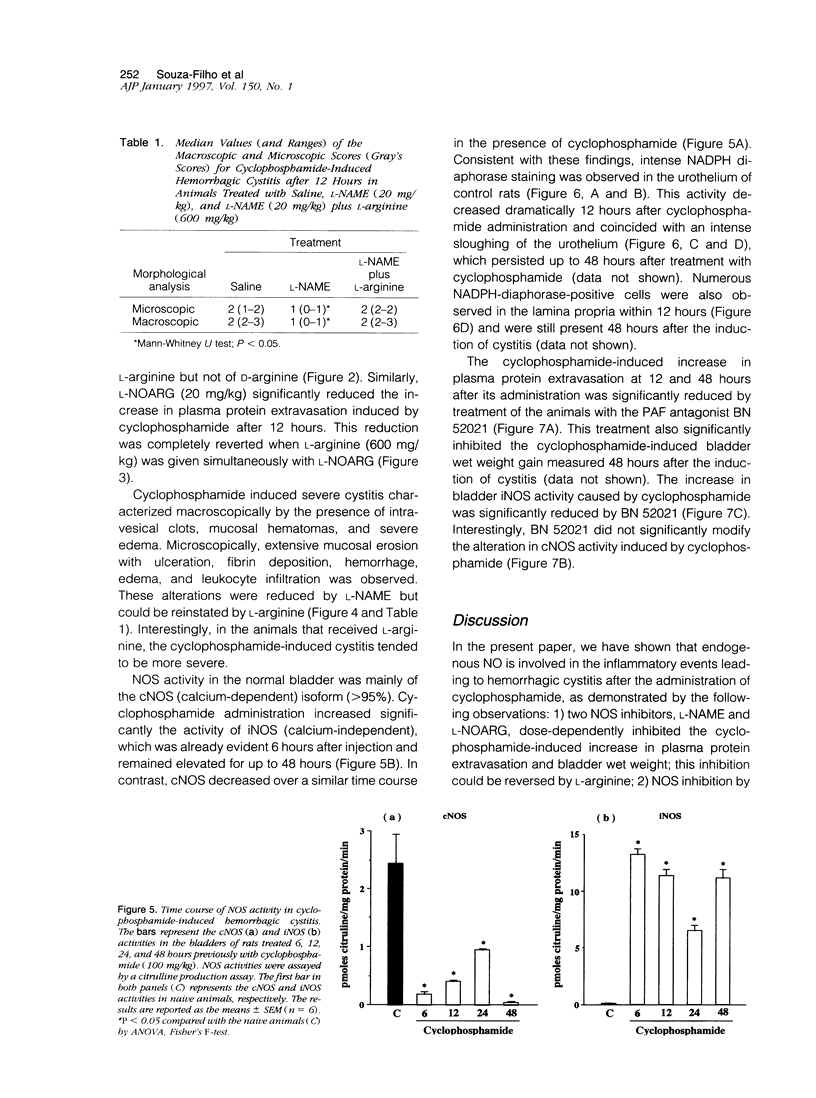
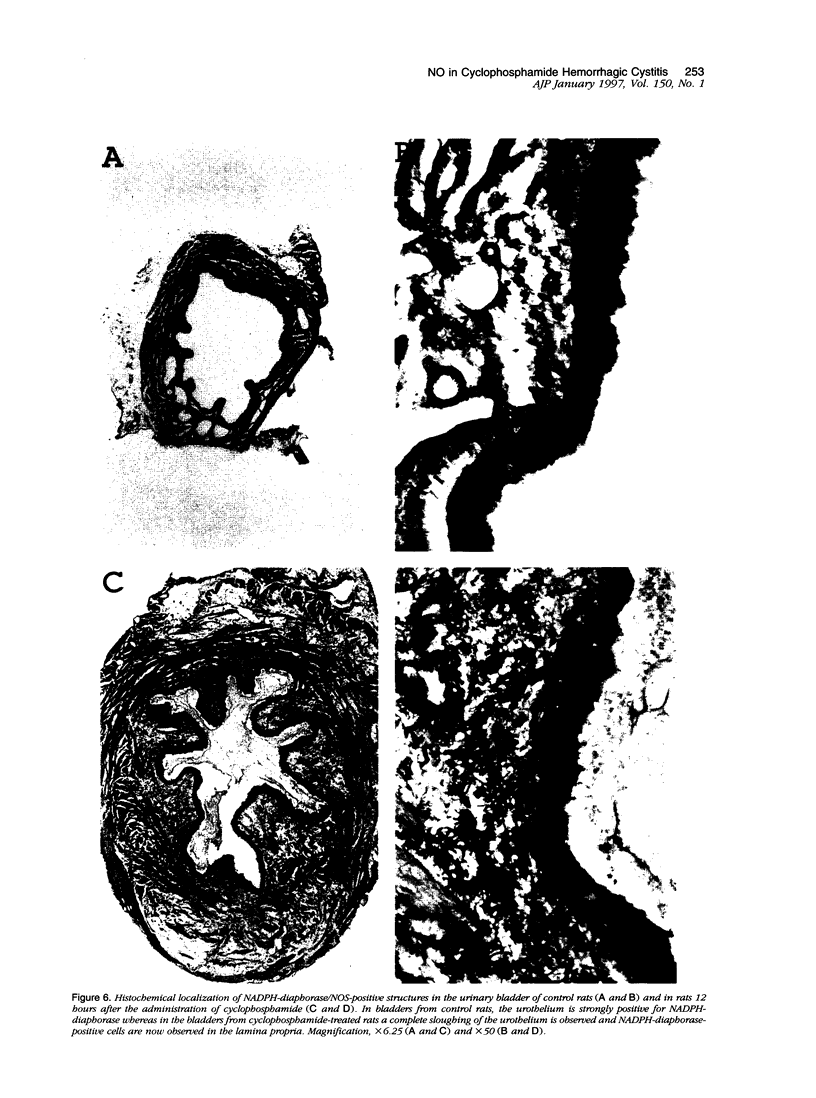
The involvement of nitric oxide (NO) and the potential modulation of NO synthase (NOS) activity by platelet-activating factor were investigated in a rat model of cyclophosphamide-induced hemorrhagic cystitis. Male Wistar rats received a single intraperitoneal injection of cyclophosphamide, and cystitis was evaluated 6, 12, 24, 48, and 72 hours later by determining the changes in bladder wet weight and plasma protein extravasation and the macro- and microscopic morphological alterations. In addition, NOS activity and NADPH-diaphorase histochemistry were studied in bladder tissues. Normal bladders showed extensive NADPH-diaphorase staining and a high level of constitutive NOS whereas the activity of inducible NOS was almost undetectable. Cyclophosphamide dose- and time-dependently increased the bladder wet weight and bladder plasma protein extravasation. These events were accompanied at a microscopic level by urothelial necrosis, sloughing, ulceration, hemorrhage, and leukocyte infiltration. Cyclophosphamide also increased the levels of inducible NOS but reduced those of constitutive NOS. The NOS inhibitors L-NG-nitroarginine methyl ester and L-NG-nitroarginine significantly reduced the cyclophosphamide-induced plasma protein extravasation and urothelial damage. This reduction was completely reversed by L-arginine but not by D-arginine. The administration of the platelet-activating factor antagonist BN 52021 decreased the cyclophosphamide-induced plasma protein extravasation as well as the rise in inducible NOS activity but had no effect on the fall in constitutive NOS activity. These results suggest that endogenous NO participates in the urothelial damage and in the inflammatory events leading to cyclophosphamide-induced hemorrhagic cystitis. Platelet-activating factor also seems to be involved in the pathogenesis of this condition, possibly by inducing NOS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antunes E., Mariano M., Cirino G., Levi S., de Nucci G. Pharmacological characterization of polycation-induced rat hind-paw oedema. Br J Pharmacol. 1990 Dec;101(4):986–990. doi: 10.1111/j.1476-5381.1990.tb14193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assreuy J., Cunha F. Q., Liew F. Y., Moncada S. Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol. 1993 Mar;108(3):833–837. doi: 10.1111/j.1476-5381.1993.tb12886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Cox P. J. Cyclophosphamide cystitis--identification of acrolein as the causative agent. Biochem Pharmacol. 1979 Jul 1;28(13):2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- Droller M. J., Saral R., Santos G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982 Sep;20(3):256–258. doi: 10.1016/0090-4295(82)90633-1. [DOI] [PubMed] [Google Scholar]
- Estrada C., Gómez C., Martín C., Moncada S., González C. Nitric oxide mediates tumor necrosis factor-alpha cytotoxicity in endothelial cells. Biochem Biophys Res Commun. 1992 Jul 15;186(1):475–482. doi: 10.1016/s0006-291x(05)80832-0. [DOI] [PubMed] [Google Scholar]
- Farrell A. J., Blake D. R., Palmer R. M., Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992 Nov;51(11):1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foad B. S., Hess E. V. Urinary bladder complications with cyclophosphamide therapy. Arch Intern Med. 1976 May;136(5):616–619. [PubMed] [Google Scholar]
- Förstermann U., Schmidt H. H., Pollock J. S., Sheng H., Mitchell J. A., Warner T. D., Nakane M., Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991 Oct 24;42(10):1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- Gomes T. N., Santos C. C., Souza-Filho M. V., Cunha F. Q., Ribeiro R. A. Participation of TNF-alpha and IL-1 in the pathogenesis of cyclophosphamide-induced hemorrhagic cystitis. Braz J Med Biol Res. 1995 Oct;28(10):1103–1108. [PubMed] [Google Scholar]
- Gray K. J., Engelmann U. H., Johnson E. H., Fishman I. J. Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy. J Urol. 1986 Aug;136(2):497–500. doi: 10.1016/s0022-5347(17)44929-9. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Vincent S. R. Histochemical characterization of neuronal NADPH-diaphorase. J Histochem Cytochem. 1989 May;37(5):653–661. doi: 10.1177/37.5.2703701. [DOI] [PubMed] [Google Scholar]
- Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992 Feb 11;211(2):177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- Ialenti A., Moncada S., Di Rosa M. Modulation of adjuvant arthritis by endogenous nitric oxide. Br J Pharmacol. 1993 Oct;110(2):701–706. doi: 10.1111/j.1476-5381.1993.tb13868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan W. G. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5'-diphosphate and bradykinin. Inflammation. 1992 Aug;16(4):295–305. doi: 10.1007/BF00917622. [DOI] [PubMed] [Google Scholar]
- Middleton S. J., Shorthouse M., Hunter J. O. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993 Feb 20;341(8843):465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Sadowska-Krowicka H., Chotinaruemol S., Kakkis J. L., Clark D. A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993 Jan;264(1):11–16. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- PHILIPS F. S., STERNBERG S. S., CRONIN A. P., VIDAL P. M. Cyclophosphamide and urinary bladder toxicity. Cancer Res. 1961 Dec;21:1577–1589. [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Snyder F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am J Physiol. 1990 Nov;259(5 Pt 1):C697–C708. doi: 10.1152/ajpcell.1990.259.5.C697. [DOI] [PubMed] [Google Scholar]
- Stillwell T. J., Benson R. C., Jr Cyclophosphamide-induced hemorrhagic cystitis. A review of 100 patients. Cancer. 1988 Feb 1;61(3):451–457. doi: 10.1002/1097-0142(19880201)61:3<451::aid-cncr2820610308>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Szabó C., Wu C. C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res. 1993 Dec;73(6):991–999. doi: 10.1161/01.res.73.6.991. [DOI] [PubMed] [Google Scholar]
- Wei X. Q., Charles I. G., Smith A., Ure J., Feng G. J., Huang F. P., Xu D., Muller W., Moncada S., Liew F. Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995 Jun 1;375(6530):408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]