Abstract
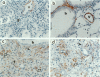
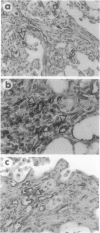
We have previously reported that transfer to rat lung of the granulocyte-macrophage colony-stimulating factor (GM-CSF) gene leads to high expression of GM-CSF between days 1 and 4 and granulation tissue formation followed by an irreversible fibrotic response starting from day 12 onward. In the current study, we investigated the underlying mechanisms. We found that GM-CSF overexpression did not enhance production of tumor necrosis factor-alpha in a significant manner at any time after GM-CSF gene transfer. However, the content of transforming growth factor-beta 1 in bronchoalveolar lavage fluid was markedly induced at day 4 and appeared to be maximal around day 7 and remained high at day 12. Macrophages purified from bronchoalveolar lavage fluid 7 days after GM-CSF gene transfer spontaneously released significant quantities of transforming growth factor-beta 1 protein in vitro. After peak transforming growth factor-beta 1 production was the emergence of alpha-smooth muscle actin-rich myofibroblasts. Accumulation of these cells was most prominent at day 12 within the granulation tissues and they were still present in fibrotic areas between days 12 and 24 and diminished markedly afterward. Thus, we provide the first in vivo evidence that tumor necrosis factor-alpha may be dissociated from participation in a fibrotic process in the lung and GM-CSF may play a more direct role in pulmonary fibrogenesis at least in part through its capability to induce transforming growth factor-beta 1 in macrophages and the subsequent emergence of myofibroblast phenotypes. This GM-CSF transgene lung model is useful for a stepwise dissection of both cellular and molecular events involved in pulmonary fibrosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Wang J. M., Defilippi P., Turrini F., Sanavio F., Edgell C. J., Aglietta M., Arese P., Mantovani A. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989 Feb 2;337(6206):471–473. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol. 1990 Oct;259(4 Pt 1):L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Desmoulière A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int. 1995 May;19(5):471–476. doi: 10.1006/cbir.1995.1090. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991 Jul 22;285(2):199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Jordana M., Cox G. Cytokines and pulmonary fibrosis. Thorax. 1993 Sep;48(9):931–935. doi: 10.1136/thx.48.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Keshav S., Stein M. BCG-induced granuloma formation in murine tissues. Immunobiology. 1994 Oct;191(4-5):369–377. doi: 10.1016/S0171-2985(11)80442-0. [DOI] [PubMed] [Google Scholar]
- Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993 Dec;92(6):2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Desmouliere A., Pache J. C., Redard M., Gabbiani G. Cytoskeletal protein modulation in pulmonary alveolar myofibroblasts during idiopathic pulmonary fibrosis. Possible role of transforming growth factor beta and tumor necrosis factor alpha. Am J Respir Crit Care Med. 1995 Dec;152(6 Pt 1):2163–2169. doi: 10.1164/ajrccm.152.6.8520791. [DOI] [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Flanders K. C., Unruh H. TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Respir Cell Mol Biol. 1996 Feb;14(2):131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991 Aug;5(2):155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- Khalil N., Whitman C., Zuo L., Danielpour D., Greenberg A. Regulation of alveolar macrophage transforming growth factor-beta secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J Clin Invest. 1993 Oct;92(4):1812–1818. doi: 10.1172/JCI116771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991 Jan;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- McDonald J. A. Idiopathic pulmonary fibrosis. A paradigm for lung injury and repair. Chest. 1991 Mar;99(3 Suppl):87S–93S. doi: 10.1378/chest.99.3_supplement.87s. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y., Araki K., Vesin C., Garcia I., Kapanci Y., Whitsett J. A., Piguet P. F., Vassalli P. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995 Jul;96(1):250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue T. D., Brody A. R. Distribution of transforming growth factor-beta 1, fibronectin, and smooth muscle actin in asbestos-induced pulmonary fibrosis in rats. J Histochem Cytochem. 1994 Aug;42(8):1061–1070. doi: 10.1177/42.8.8027525. [DOI] [PubMed] [Google Scholar]
- Phan S. H., Kunkel S. L. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res. 1992 Jan-Mar;18(1):29–43. doi: 10.3109/01902149209020649. [DOI] [PubMed] [Google Scholar]
- Piguet P. F. Cytokines involved in pulmonary fibrosis. Int Rev Exp Pathol. 1993;34(Pt B):173–181. doi: 10.1016/b978-0-12-364935-5.50017-1. [DOI] [PubMed] [Google Scholar]
- Raghow R. Role of transforming growth factor-beta in repair and fibrosis. Chest. 1991 Mar;99(3 Suppl):61S–65S. doi: 10.1378/chest.99.3_supplement.61s. [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Sappino A. P., Gabbiani G. Locally applied GM-CSF induces the accumulation of alpha-smooth muscle actin containing myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(2):73–82. doi: 10.1007/BF02899530. [DOI] [PubMed] [Google Scholar]
- Vyalov S. L., Gabbiani G., Kapanci Y. Rat alveolar myofibroblasts acquire alpha-smooth muscle actin expression during bleomycin-induced pulmonary fibrosis. Am J Pathol. 1993 Dec;143(6):1754–1765. [PMC free article] [PubMed] [Google Scholar]
- Vyalov S., Desmoulière A., Gabbiani G. GM-CSF-induced granulation tissue formation: relationships between macrophage and myofibroblast accumulation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(4):231–239. doi: 10.1007/BF02899267. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T., Feng L., Masliah E., Ruppe M. D., Lee H. S., Toggas S. M., Rockenstein E. M., Mucke L. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol. 1995 Jul;147(1):53–67. [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Braciak T., Ohkawara Y., Sallenave J. M., Foley R., Sime P. J., Jordana M., Graham F. L., Gauldie J. Gene transfer for cytokine functional studies in the lung: the multifunctional role of GM-CSF in pulmonary inflammation. J Leukoc Biol. 1996 Apr;59(4):481–488. doi: 10.1002/jlb.59.4.481. [DOI] [PubMed] [Google Scholar]
- Xing Z., Jordana M., Kirpalani H., Driscoll K. E., Schall T. J., Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994 Feb;10(2):148–153. doi: 10.1165/ajrcmb.10.2.8110470. [DOI] [PubMed] [Google Scholar]
- Xing Z., Kirpalani H., Torry D., Jordana M., Gauldie J. Polymorphonuclear leukocytes as a significant source of tumor necrosis factor-alpha in endotoxin-challenged lung tissue. Am J Pathol. 1993 Oct;143(4):1009–1015. [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Ohkawara Y., Jordana M., Graham F., Gauldie J. Transfer of granulocyte-macrophage colony-stimulating factor gene to rat lung induces eosinophilia, monocytosis, and fibrotic reactions. J Clin Invest. 1996 Feb 15;97(4):1102–1110. doi: 10.1172/JCI118503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Flanders K. C., Phan S. H. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1995 Aug;147(2):352–361. [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Rekhter M. D., Gordon D., Phan S. H. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994 Jul;145(1):114–125. [PMC free article] [PubMed] [Google Scholar]