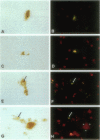
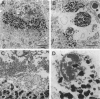
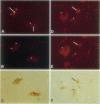
Abstract
In Parkinson's disease and other Lewy-body-associated disorders, the substantia nigra pars compacta undergoes degeneration, but the mechanism of cell death has not been previously described. The substantia nigra of normal and Alzheimer's disease cases were compared with substantia nigra from patients with Lewy-body-associated disorders (Parkinson's disease, concomitant Alzheimer's/Parkinson's disease, and diffuse Lewy body disease) using in situ end labeling to detect fragmented DNA. In situ end-labeled neurons demonstrated changes resembling apoptosis: nuclear condensation, chromatin fragmentation, and formation of apoptotic-like bodies. Ultrastructural analysis confirmed nuclear condensation and formation of apoptotic-like bodies. Apoptotic-like changes were seen in the substantia nigra of both normal and diseased cases; concomitant Alzheimer's/Parkinson's disease and diffuse Lewy body disease cases had significantly higher amounts of apoptotic-like changes than normal controls or Alzheimer patients. The finding of neuronal death by apoptosis may have relevance for the development of new treatment strategies for Parkinson's disease and related disorders.
Full text
PDF


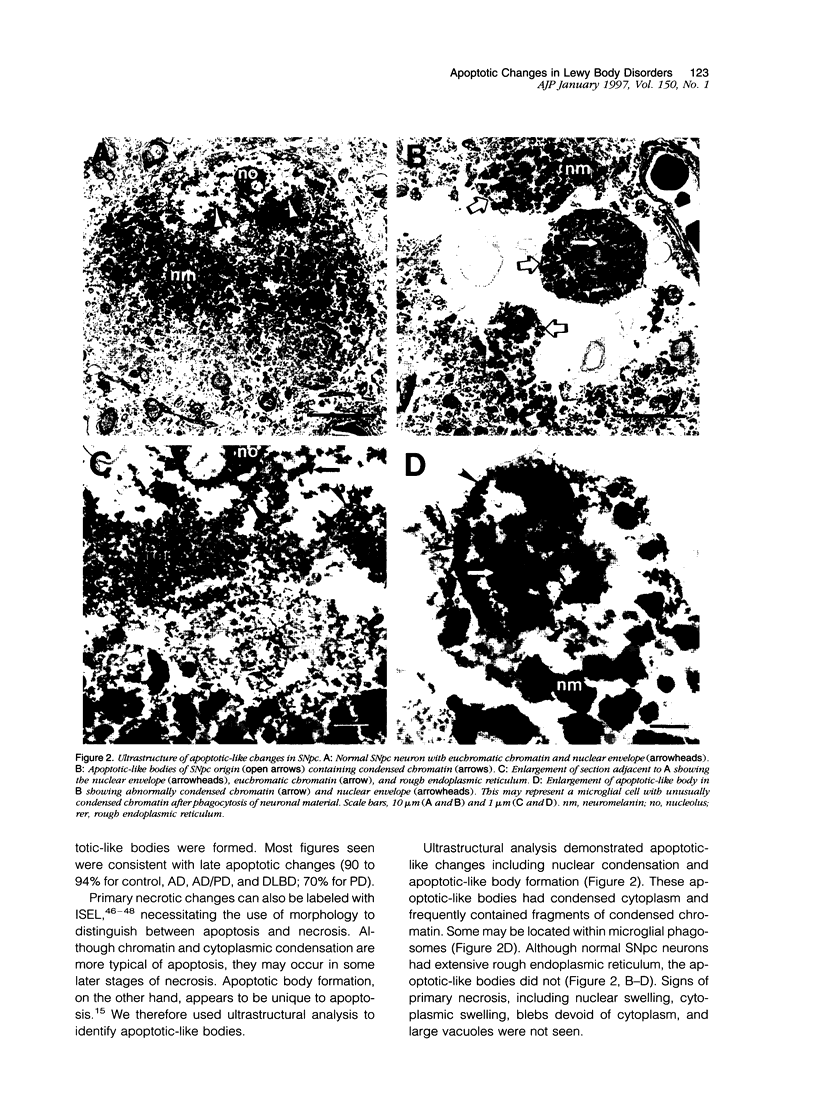
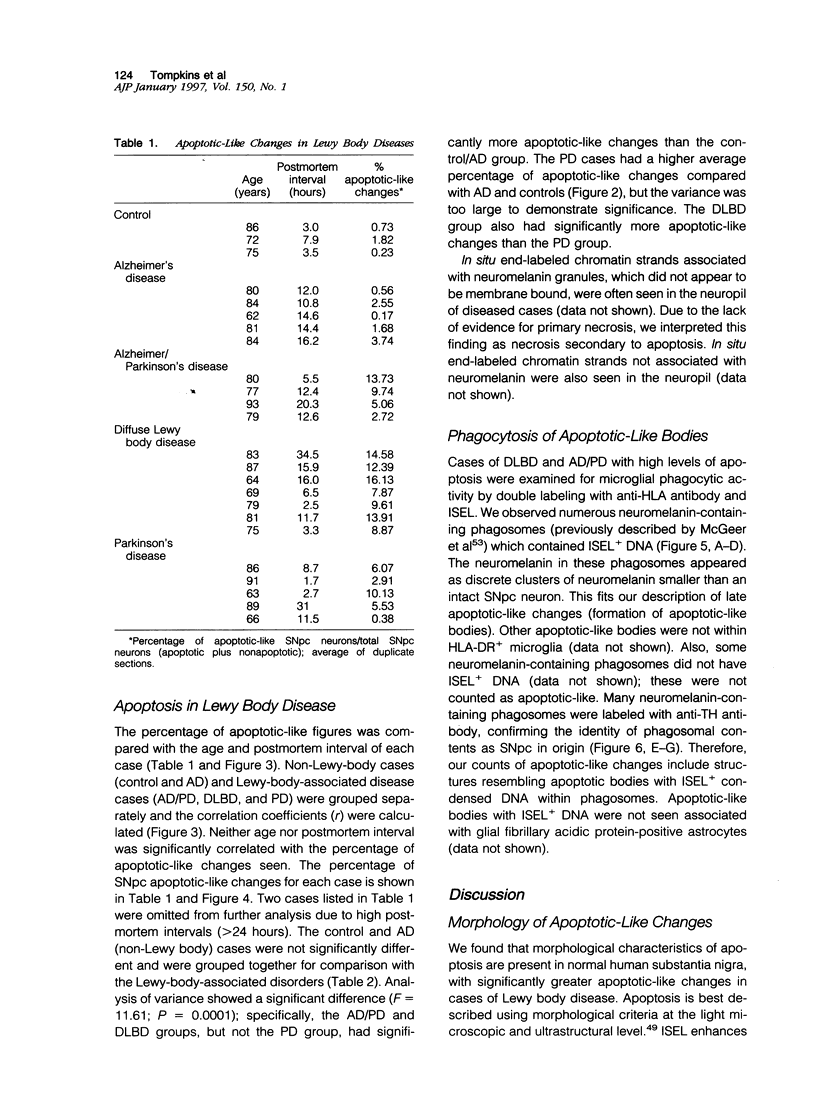
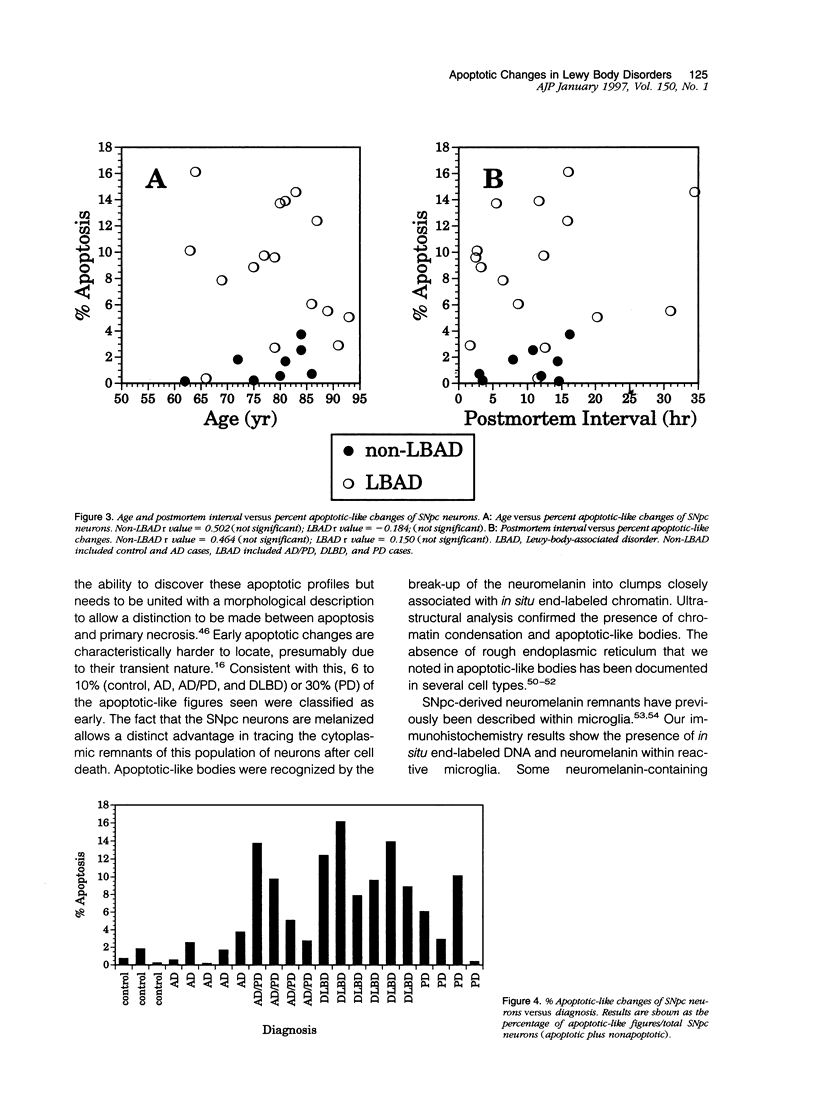
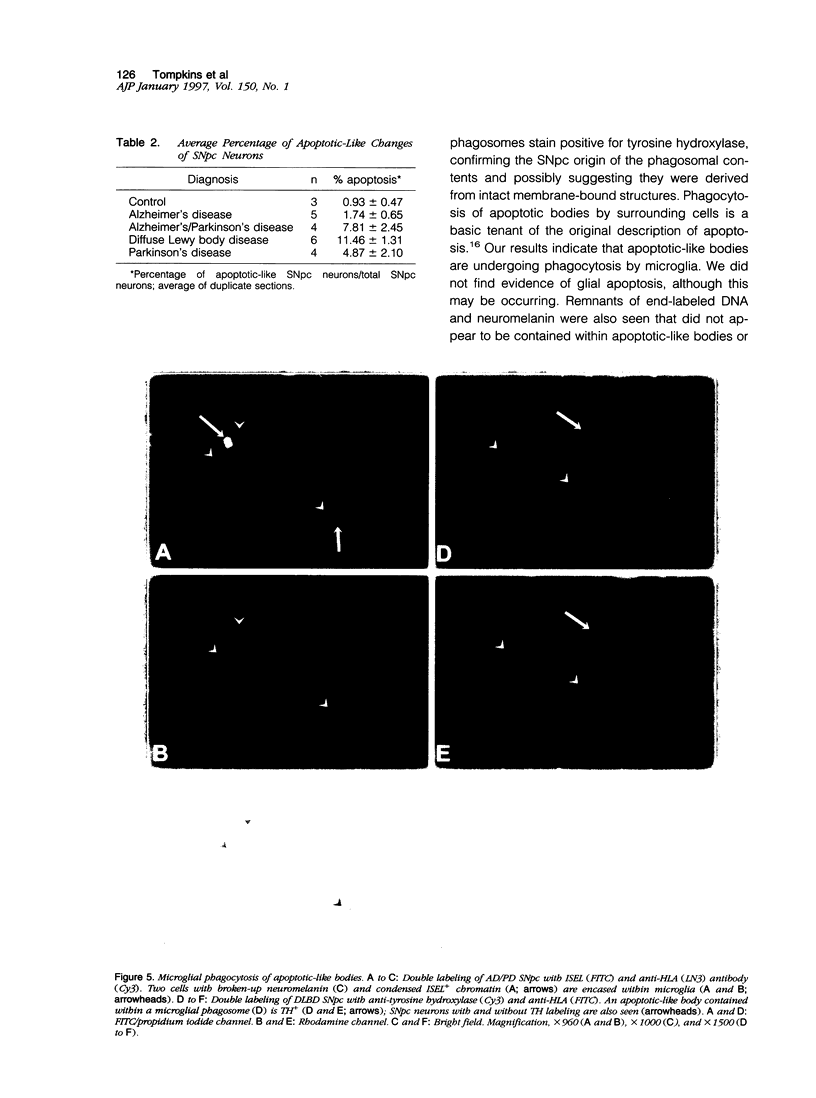





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp T. E., Wyatt S., Paterson H. F., Davies A. M. The proto-oncogene bcl-2 can selectively rescue neurotrophic factor-dependent neurons from apoptosis. Cell. 1993 Apr 23;73(2):295–307. doi: 10.1016/0092-8674(93)90230-n. [DOI] [PubMed] [Google Scholar]
- Ansari B., Coates P. J., Greenstein B. D., Hall P. A. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993 May;170(1):1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- Beck K. D., Valverde J., Alexi T., Poulsen K., Moffat B., Vandlen R. A., Rosenthal A., Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995 Jan 26;373(6512):339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- Bojinov S. Encephalitis with acute Parkinsonian syndrome and bilateral inflammatory necrosis of the substantia nigra. J Neurol Sci. 1971 Apr;12(4):383–415. doi: 10.1016/0022-510x(71)90109-2. [DOI] [PubMed] [Google Scholar]
- Boller F., Mizutani T., Roessmann U., Gambetti P. Parkinson disease, dementia, and Alzheimer disease: clinicopathological correlations. Ann Neurol. 1980 Apr;7(4):329–335. doi: 10.1002/ana.410070408. [DOI] [PubMed] [Google Scholar]
- Buja L. M., Eigenbrodt M. L., Eigenbrodt E. H. Apoptosis and necrosis. Basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993 Dec;117(12):1208–1214. [PubMed] [Google Scholar]
- Burkhardt C. R., Filley C. M., Kleinschmidt-DeMasters B. K., de la Monte S., Norenberg M. D., Schneck S. A. Diffuse Lewy body disease and progressive dementia. Neurology. 1988 Oct;38(10):1520–1528. doi: 10.1212/wnl.38.10.1520. [DOI] [PubMed] [Google Scholar]
- Clarke P. G. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 1990;181(3):195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- Daniel S. E., Lees A. J. Parkinson's Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl. 1993;39:165–172. [PubMed] [Google Scholar]
- Deckwerth T. L., Johnson E. M., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993 Dec;123(5):1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson D. W., Crystal H., Mattiace L. A., Kress Y., Schwagerl A., Ksiezak-Reding H., Davies P., Yen S. H. Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol. 1989;78(6):572–584. doi: 10.1007/BF00691284. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Ruan D., Crystal H., Mark M. H., Davies P., Kress Y., Yen S. H. Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology. 1991 Sep;41(9):1402–1409. doi: 10.1212/wnl.41.9.1402. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Faull R. L., Lawlor P., Beilharz E. J., Singleton K., Walker E. B., Mee E. In situ evidence for DNA fragmentation in Huntington's disease striatum and Alzheimer's disease temporal lobes. Neuroreport. 1995 May 9;6(7):1053–1057. doi: 10.1097/00001756-199505090-00026. [DOI] [PubMed] [Google Scholar]
- Duvoisin R. C., Golbe L. I. Toward a definition of Parkinson's disease. Neurology. 1989 May;39(5):746–746. doi: 10.1212/wnl.39.5.746. [DOI] [PubMed] [Google Scholar]
- Fearnley J. M., Lees A. J. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991 Oct;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Förstl H., Burns A., Luthert P., Cairns N., Levy R. The Lewy-body variant of Alzheimer's disease. Clinical and pathological findings. Br J Psychiatry. 1993 Mar;162:385–392. doi: 10.1192/bjp.162.3.385. [DOI] [PubMed] [Google Scholar]
- Galli G., Fratelli M. Activation of apoptosis by serum deprivation in a teratocarcinoma cell line: inhibition by L-acetylcarnitine. Exp Cell Res. 1993 Jan;204(1):54–60. doi: 10.1006/excr.1993.1008. [DOI] [PubMed] [Google Scholar]
- Galloway P. G., Bergeron C., Perry G. The presence of tau distinguishes Lewy bodies of diffuse Lewy body disease from those of idiopathic Parkinson disease. Neurosci Lett. 1989 May 22;100(1-3):6–10. doi: 10.1016/0304-3940(89)90651-4. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb W. R., Esiri M. M., Lees A. J. Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia). Brain. 1987 Oct;110(Pt 5):1131–1153. doi: 10.1093/brain/110.5.1131. [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Lees A. J. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991 May;54(5):388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb W. R., Luthert P. J., Janota I., Lantos P. L. Cortical Lewy body dementia: clinical features and classification. J Neurol Neurosurg Psychiatry. 1989 Feb;52(2):185–192. doi: 10.1136/jnnp.52.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb W. R. Neuropathology in movement disorders. J Neurol Neurosurg Psychiatry. 1989 Jun;Suppl:55–67. doi: 10.1136/jnnp.52.suppl.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Schmied M., Giegerich G., Breitschopf H., Hartung H. P., Toyka K. V., Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994 Aug;71(2):219–225. [PubMed] [Google Scholar]
- Hansen L., Salmon D., Galasko D., Masliah E., Katzman R., DeTeresa R., Thal L., Pay M. M., Hofstetter R., Klauber M. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology. 1990 Jan;40(1):1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- Hartley A., Stone J. M., Heron C., Cooper J. M., Schapira A. H. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson's disease. J Neurochem. 1994 Nov;63(5):1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. Defining apoptosis. Am J Pathol. 1995 Jan;146(1):16–19. [PMC free article] [PubMed] [Google Scholar]
- Ince P., Irving D., MacArthur F., Perry R. H. Quantitative neuropathological study of Alzheimer-type pathology in the hippocampus: comparison of senile dementia of Alzheimer type, senile dementia of Lewy body type, Parkinson's disease and non-demented elderly control patients. J Neurol Sci. 1991 Dec;106(2):142–152. doi: 10.1016/0022-510x(91)90251-2. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kosaka K. Diffuse Lewy body disease in Japan. J Neurol. 1990 Jun;237(3):197–204. doi: 10.1007/BF00314594. [DOI] [PubMed] [Google Scholar]
- Larsson B. S. Interaction between chemicals and melanin. Pigment Cell Res. 1993 Jun;6(3):127–133. doi: 10.1111/j.1600-0749.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H., Bancher C., Breitschopf H., Wegiel J., Bobinski M., Jellinger K., Wisniewski H. M. Cell death in Alzheimer's disease evaluated by DNA fragmentation in situ. Acta Neuropathol. 1995;89(1):35–41. doi: 10.1007/BF00294257. [DOI] [PubMed] [Google Scholar]
- Lennox G., Lowe J., Byrne E. J., Landon M., Mayer R. J., Godwin-Austen R. B. Diffuse Lewy body disease. Lancet. 1989 Feb 11;1(8633):323–324. doi: 10.1016/s0140-6736(89)91328-7. [DOI] [PubMed] [Google Scholar]
- Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993 May 21;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lippa C. F., Smith T. W., Swearer J. M. Alzheimer's disease and Lewy body disease: a comparative clinicopathological study. Ann Neurol. 1994 Jan;35(1):81–88. doi: 10.1002/ana.410350113. [DOI] [PubMed] [Google Scholar]
- Macaya A., Munell F., Gubits R. M., Burke R. E. Apoptosis in substantia nigra following developmental striatal excitotoxic injury. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8117–8121. doi: 10.1073/pnas.91.17.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Marcyniuk B. Monoaminergic neurotransmitter systems in presenile Alzheimer's disease and in senile dementia of Alzheimer type. Clin Neuropathol. 1984 Sep-Oct;3(5):199–205. [PubMed] [Google Scholar]
- Martinou J. C., Dubois-Dauphin M., Staple J. K., Rodriguez I., Frankowski H., Missotten M., Albertini P., Talabot D., Catsicas S., Pietra C. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994 Oct;13(4):1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Akiyama H., McGeer E. G. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988 Oct;24(4):574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Boyes B. E., McGeer E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988 Aug;38(8):1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Mirra S. S., Hart M. N., Terry R. D. Making the diagnosis of Alzheimer's disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993 Feb;117(2):132–144. [PubMed] [Google Scholar]
- Mirra S. S., Heyman A., McKeel D., Sumi S. M., Crain B. J., Brownlee L. M., Vogel F. S., Hughes J. P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Morris J. C., Drazner M., Fulling K., Grant E. A., Goldring J. Clinical and pathological aspects of parkinsonism in Alzheimer's disease. A role for extranigral factors? Arch Neurol. 1989 Jun;46(6):651–657. doi: 10.1001/archneur.1989.00520420071025. [DOI] [PubMed] [Google Scholar]
- Mundle S. D., Raza A. The two in situ techniques do not differentiate between apoptosis and necrosis but rather reveal distinct patterns of DNA fragmentation in apoptosis. Lab Invest. 1995 May;72(5):611–613. [PubMed] [Google Scholar]
- O'Connor T. M., Wyttenbach C. R. Cell death in the embryonic chick spinal cord. J Cell Biol. 1974 Feb;60(2):448–459. doi: 10.1083/jcb.60.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow C. W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993 Nov;16(11):439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Perry R. H., Irving D., Blessed G., Fairbairn A., Perry E. K. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci. 1990 Feb;95(2):119–139. doi: 10.1016/0022-510x(90)90236-g. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Roberts B. Effect of postmortem interval on in situ end-labeling of DNA oligonucleosomes. J Neuropathol Exp Neurol. 1995 Nov;54(6):761–765. doi: 10.1097/00005072-199511000-00002. [DOI] [PubMed] [Google Scholar]
- Pilar G., Landmesser L. Ultrastructural differences during embryonic cell death in normal and peripherally deprived ciliary ganglia. J Cell Biol. 1976 Feb;68(2):339–356. doi: 10.1083/jcb.68.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C., Hedreen J. C., Price D. L., Koliatsos V. E. Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J Neurosci. 1995 May;15(5 Pt 2):3775–3787. doi: 10.1523/JNEUROSCI.15-05-03775.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn N. P., Husain F. A. Parkinson's disease. Br Med J (Clin Res Ed) 1986 Aug 9;293(6543):379–382. doi: 10.1136/bmj.293.6543.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan R. R., Murphy T. H., Baraban J. M. Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem. 1994 Jan;62(1):376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990 Mar;54(3):823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Su J. H., Anderson A. J., Cummings B. J., Cotman C. W. Immunohistochemical evidence for apoptosis in Alzheimer's disease. Neuroreport. 1994 Dec 20;5(18):2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- Takeshima T., Johnston J. M., Commissiong J. W. Mesencephalic type 1 astrocytes rescue dopaminergic neurons from death induced by serum deprivation. J Neurosci. 1994 Aug;14(8):4769–4779. doi: 10.1523/JNEUROSCI.14-08-04769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac A., Lindqvist E., Lin L. F., Ogren S. O., Young D., Hoffer B. J., Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995 Jan 26;373(6512):335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Troost D., Aten J., Morsink F., de Jong J. M. Apoptosis in amyotrophic lateral sclerosis is not restricted to motor neurons. Bcl-2 expression is increased in unaffected post-central gyrus. Neuropathol Appl Neurobiol. 1995 Dec;21(6):498–504. doi: 10.1111/j.1365-2990.1995.tb01096.x. [DOI] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Wolvetang E. J., Johnson K. L., Krauer K., Ralph S. J., Linnane A. W. Mitochondrial respiratory chain inhibitors induce apoptosis. FEBS Lett. 1994 Feb 14;339(1-2):40–44. doi: 10.1016/0014-5793(94)80380-3. [DOI] [PubMed] [Google Scholar]
- Wood K. A., Dipasquale B., Youle R. J. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993 Oct;11(4):621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- Yan Q., Matheson C., Lopez O. T. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995 Jan 26;373(6512):341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y., Yamada T., Asanuma K., Asahi T. Apoptosis related antigen, Le(Y) and nick-end labeling are positive in spinal motor neurons in amyotrophic lateral sclerosis. Acta Neuropathol. 1994;88(3):207–211. doi: 10.1007/BF00293395. [DOI] [PubMed] [Google Scholar]
- Ziv I., Melamed E., Nardi N., Luria D., Achiron A., Offen D., Barzilai A. Dopamine induces apoptosis-like cell death in cultured chick sympathetic neurons--a possible novel pathogenetic mechanism in Parkinson's disease. Neurosci Lett. 1994 Mar 28;170(1):136–140. doi: 10.1016/0304-3940(94)90258-5. [DOI] [PubMed] [Google Scholar]