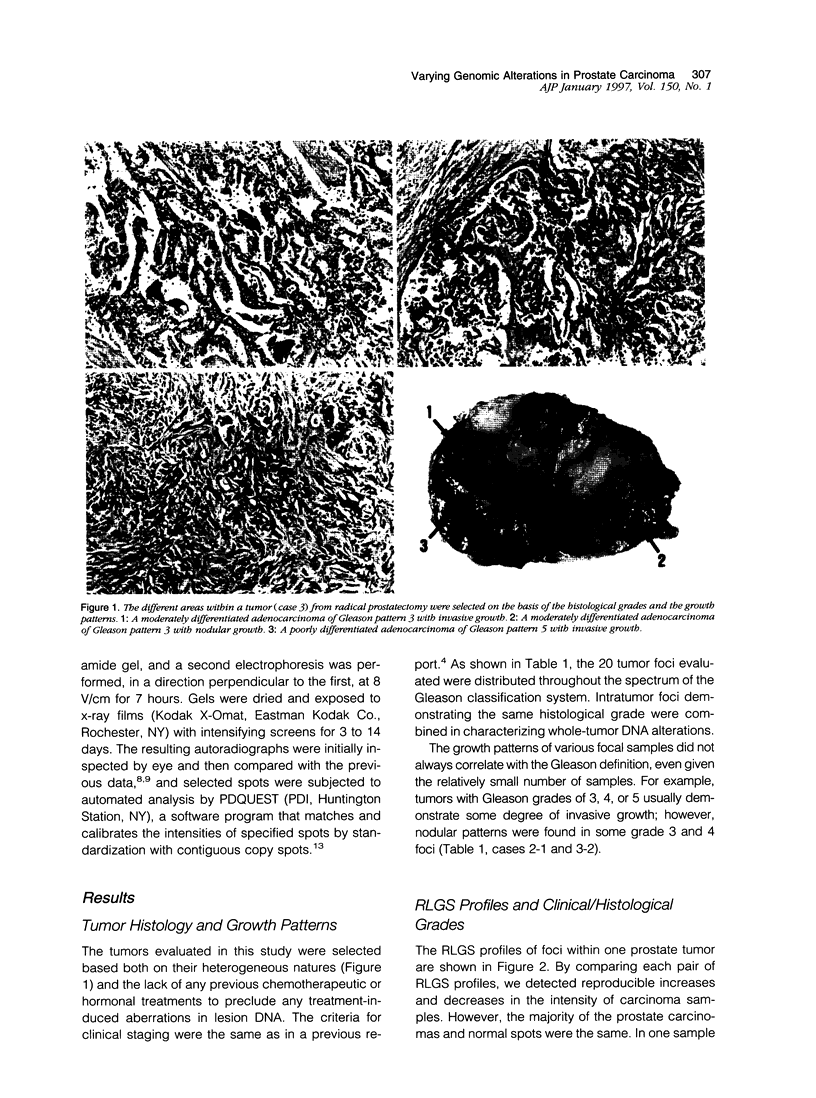
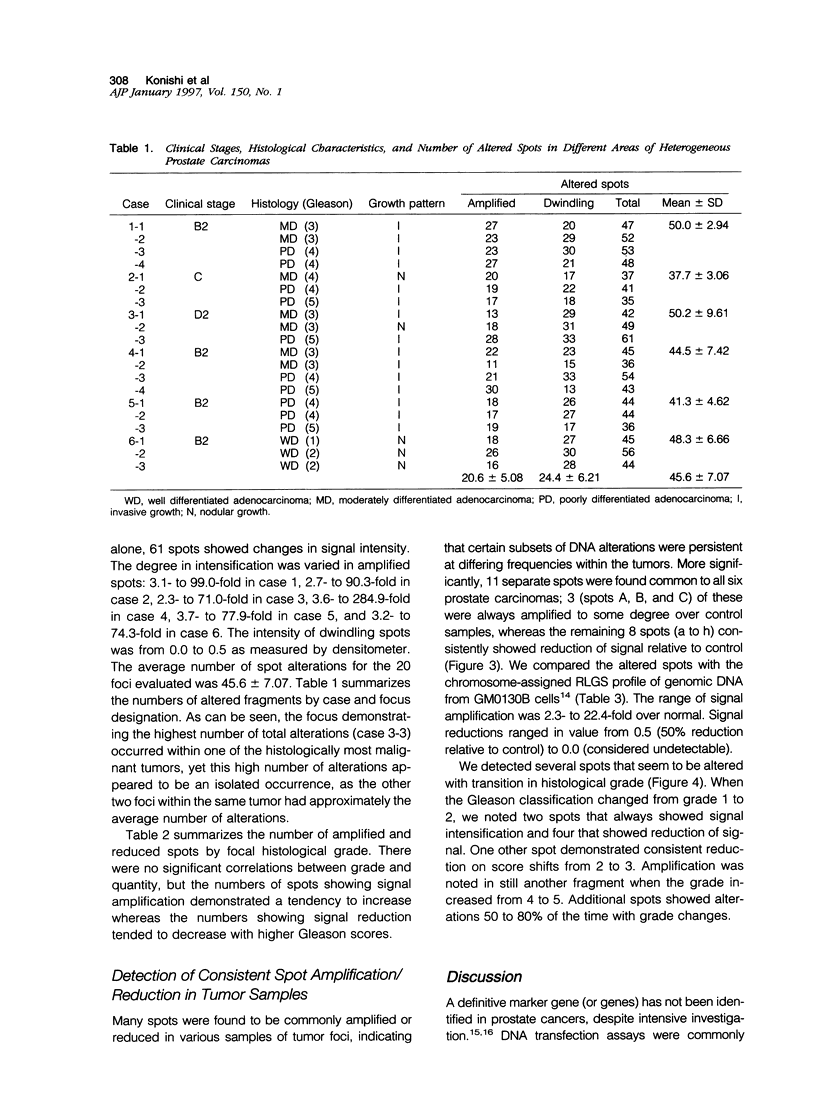
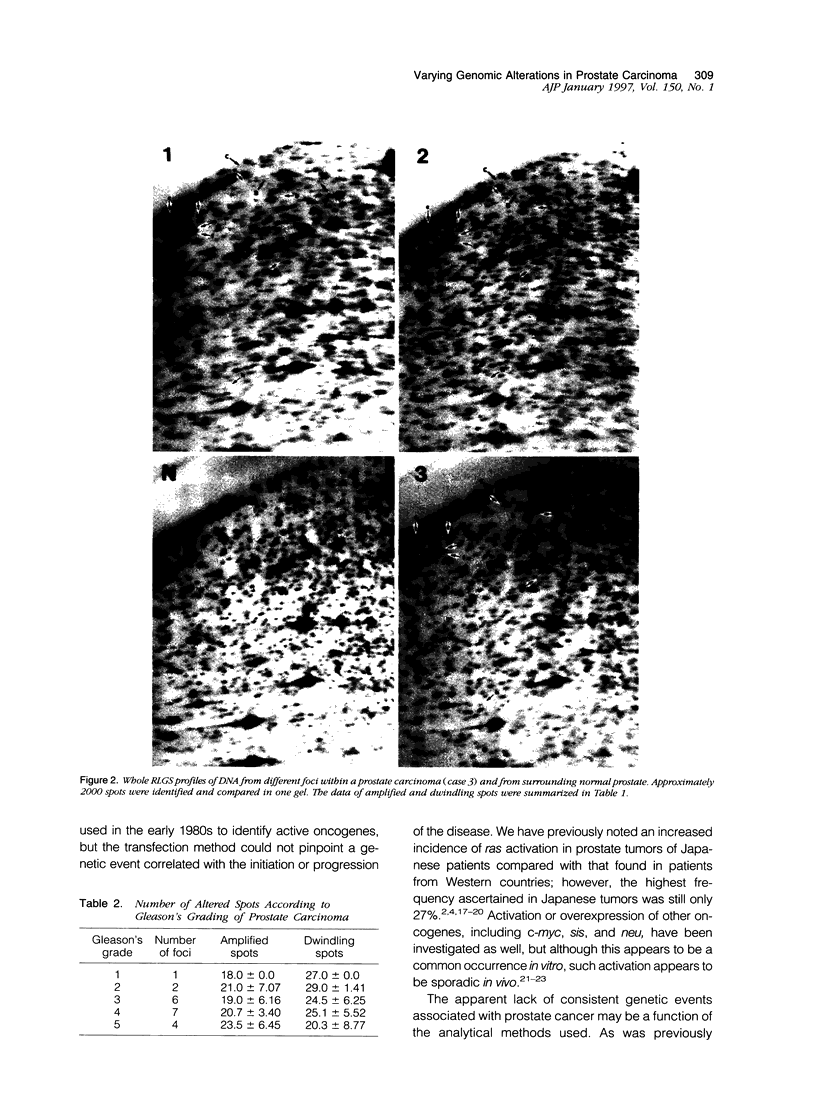
Abstract
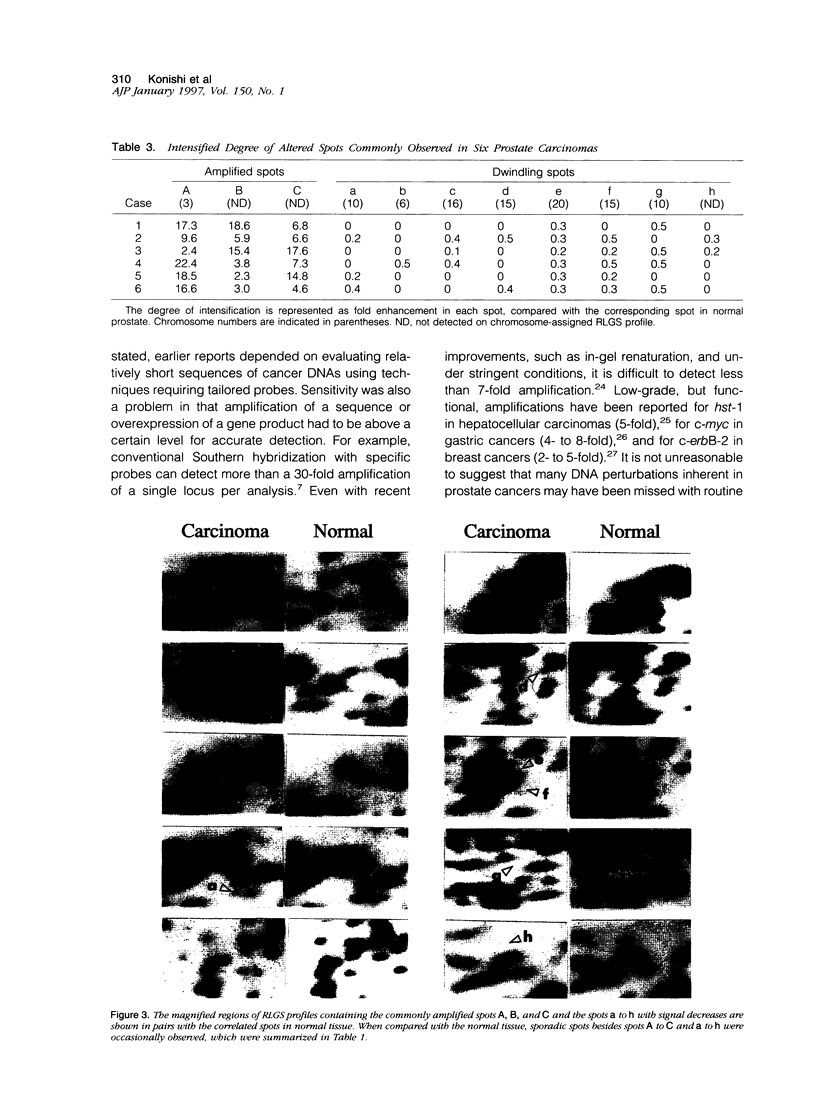
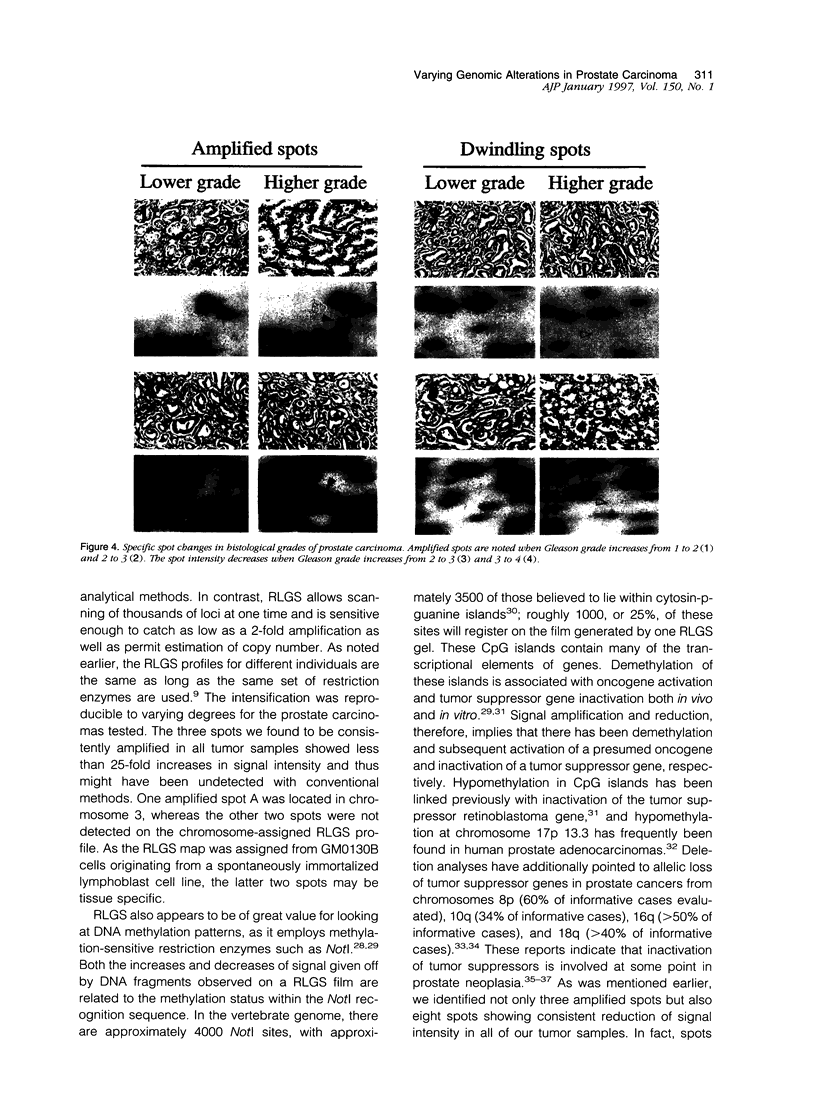
Alterations in the genomic DNAs of six heterogeneous prostate carcinomas, as well as that of individual and histologically distinct foci within the tumors, were examined using restriction landmark genomic scanning, a method employing two-dimensional gel analysis of a large number of DNA fragments generated by digestion with highly specific endonucleases. Upon autoradiographic imaging, these fragments appear as spots of varying intensity and location specific for each sample. In our study, comparison of cancer DNAs against normal prostate DNA controls yielded alterations in at least 35 spots. Despite differences in the histological grading of tumors, 3 spots common to all tumor samples showed consistent amplification of intensity and 8 other common spots demonstrated consistent reduction of intensity when compared with control. In addition, spot alterations occurred between histologically identical foci isolated from within single tumors. We suggest that these spot changes detected in DNA profiles generated by restriction landmark genomic scanning reflect aberrations in as yet unidentified oncogenes and tumor suppressor genes and indicate that prostate cancer is not only histologically heterogeneous and multifocal but also genetically multicentric.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastacky S. I., Wojno K. J., Walsh P. C., Carmichael M. J., Epstein J. I. Pathological features of hereditary prostate cancer. J Urol. 1995 Mar;153(3 Pt 2):987–992. [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. S., Epstein J. I., Isaacs W. B. ras gene mutations in human prostate cancer. Cancer Res. 1990 Nov 1;50(21):6830–6832. [PubMed] [Google Scholar]
- Carter B. S., Ewing C. M., Ward W. S., Treiger B. F., Aalders T. W., Schalken J. A., Epstein J. I., Isaacs W. B. Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8751–8755. doi: 10.1073/pnas.87.22.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert-Buck M. R., Vocke C. D., Pozzatti R. O., Duray P. H., Jennings S. B., Florence C. D., Zhuang Z., Bostwick D. G., Liotta L. A., Linehan W. M. Allelic loss on chromosome 8p12-21 in microdissected prostatic intraepithelial neoplasia. Cancer Res. 1995 Jul 15;55(14):2959–2962. [PubMed] [Google Scholar]
- Ewing C. M., Ru N., Morton R. A., Robinson J. C., Wheelock M. J., Johnson K. R., Barrett J. C., Isaacs W. B. Chromosome 5 suppresses tumorigenicity of PC3 prostate cancer cells: correlation with re-expression of alpha-catenin and restoration of E-cadherin function. Cancer Res. 1995 Nov 1;55(21):4813–4817. [PubMed] [Google Scholar]
- Fukumoto M., Shevrin D. H., Roninson I. B. Analysis of gene amplification in human tumor cell lines. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6846–6850. doi: 10.1073/pnas.85.18.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I. The QUEST system for quantitative analysis of two-dimensional gels. J Biol Chem. 1989 Mar 25;264(9):5269–5282. [PubMed] [Google Scholar]
- Gleason D. F. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966 Mar;50(3):125–128. [PubMed] [Google Scholar]
- Gumerlock P. H., Poonamallee U. R., Meyers F. J., deVere White R. W. Activated ras alleles in human carcinoma of the prostate are rare. Cancer Res. 1991 Mar 15;51(6):1632–1637. [PubMed] [Google Scholar]
- Hatada I., Hayashizaki Y., Hirotsune S., Komatsubara H., Mukai T. A genomic scanning method for higher organisms using restriction sites as landmarks. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9523–9527. doi: 10.1073/pnas.88.21.9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada I., Tokino T., Ochiya T., Matsubara K. Co-amplification of integrated hepatitis B virus DNA and transforming gene hst-1 in a hepatocellular carcinoma. Oncogene. 1988 Nov;3(5):537–540. [PubMed] [Google Scholar]
- Hirotsune S., Hatada I., Komatsubara H., Nagai H., Kuma K., Kobayakawa K., Kawara T., Nakagawara A., Fujii K., Mukai T. New approach for detection of amplification in cancer DNA using restriction landmark genomic scanning. Cancer Res. 1992 Jul 1;52(13):3642–3647. [PubMed] [Google Scholar]
- Joos S., Bergerheim U. S., Pan Y., Matsuyama H., Bentz M., du Manoir S., Lichter P. Mapping of chromosomal gains and losses in prostate cancer by comparative genomic hybridization. Genes Chromosomes Cancer. 1995 Dec;14(4):267–276. doi: 10.1002/gcc.2870140405. [DOI] [PubMed] [Google Scholar]
- Kawai J., Hirose K., Fushiki S., Hirotsune S., Ozawa N., Hara A., Hayashizaki Y., Watanabe S. Comparison of DNA methylation patterns among mouse cell lines by restriction landmark genomic scanning. Mol Cell Biol. 1994 Nov;14(11):7421–7427. doi: 10.1128/mcb.14.11.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda T., Matsushima S., Sasaki A., Danjo Y., Kakinuma M. c-myc Gene amplification in primary stomach cancer. Jpn J Cancer Res. 1985 Jul;76(7):551–554. [PubMed] [Google Scholar]
- Konishi N., Enomoto T., Buzard G., Ohshima M., Ward J. M., Rice J. M. K-ras activation and ras p21 expression in latent prostatic carcinoma in Japanese men. Cancer. 1992 May 1;69(9):2293–2299. doi: 10.1002/1097-0142(19920501)69:9<2293::aid-cncr2820690915>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Konishi N., Hiasa Y., Hayashi I., Matsuda H., Tsuzuki T., Ming T., Kitahori Y., Shiraishi T., Yatani R., Shimazaki J. p53 mutations occur in clinical, but not latent, human prostate carcinoma. Jpn J Cancer Res. 1995 Jan;86(1):57–63. doi: 10.1111/j.1349-7006.1995.tb02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi N., Hiasa Y., Matsuda H., Tao M., Tsuzuki T., Hayashi I., Kitahori Y., Shiraishi T., Yatani R., Shimazaki J. Intratumor cellular heterogeneity and alterations in ras oncogene and p53 tumor suppressor gene in human prostate carcinoma. Am J Pathol. 1995 Oct;147(4):1112–1122. [PMC free article] [PubMed] [Google Scholar]
- Lindsay S., Bird A. P. Use of restriction enzymes to detect potential gene sequences in mammalian DNA. 1987 May 28-Jun 3Nature. 327(6120):336–338. doi: 10.1038/327336a0. [DOI] [PubMed] [Google Scholar]
- Miwa W., Yashima K., Sekine T., Sekiya T. Demethylation of a repetitive DNA sequence in human cancers. Electrophoresis. 1995 Feb;16(2):227–232. doi: 10.1002/elps.1150160138. [DOI] [PubMed] [Google Scholar]
- Moul J. W., Friedrichs P. A., Lance R. S., Theune S. M., Chang E. H. Infrequent RAS oncogene mutations in human prostate cancer. Prostate. 1992;20(4):327–338. doi: 10.1002/pros.2990200407. [DOI] [PubMed] [Google Scholar]
- Nag A., Smith R. G. Amplification, rearrangement, and elevated expression of c-myc in the human prostatic carcinoma cell line LNCaP. Prostate. 1989;15(2):115–122. doi: 10.1002/pros.2990150205. [DOI] [PubMed] [Google Scholar]
- Nagai H., Hirotsune S., Komatsubara H., Hatada I., Mukai T., Hayashizaki Y., Matsubara K. Genomic analysis of human hepatocellular carcinomas using Restriction Landmark Genomic Scanning. Cancer Detect Prev. 1993;17(3):399–404. [PubMed] [Google Scholar]
- Nagai H., Ponglikitmongkol M., Mita E., Ohmachi Y., Yoshikawa H., Saeki R., Yumoto Y., Nakanishi T., Matsubara K. Aberration of genomic DNA in association with human hepatocellular carcinomas detected by 2-dimensional gel analysis. Cancer Res. 1994 Mar 15;54(6):1545–1550. [PubMed] [Google Scholar]
- Peehl D. M. Oncogenes in prostate cancer. An update. Cancer. 1993 Feb 1;71(3 Suppl):1159–1164. doi: 10.1002/1097-0142(19930201)71:3+<1159::aid-cncr2820711439>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Rinker-Schaeffer C. W., Hawkins A. L., Ru N., Dong J., Stoica G., Griffin C. A., Ichikawa T., Barrett J. C., Isaacs J. T. Differential suppression of mammary and prostate cancer metastasis by human chromosomes 17 and 11. Cancer Res. 1994 Dec 1;54(23):6249–6256. [PubMed] [Google Scholar]
- Roninson I. B. Detection and mapping of homologous, repeated and amplified DNA sequences by DNA renaturation in agarose gels. Nucleic Acids Res. 1983 Aug 25;11(16):5413–5431. doi: 10.1093/nar/11.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Toguchida J., Ohtani N., Yandell D. W., Rapaport J. M., Dryja T. P. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991 May;48(5):880–888. [PMC free article] [PubMed] [Google Scholar]
- Sakr W. A., Macoska J. A., Benson P., Grignon D. J., Wolman S. R., Pontes J. E., Crissman J. D. Allelic loss in locally metastatic, multisampled prostate cancer. Cancer Res. 1994 Jun 15;54(12):3273–3277. [PubMed] [Google Scholar]
- Sitaras N. M., Sariban E., Bravo M., Pantazis P., Antoniades H. N. Constitutive production of platelet-derived growth factor-like proteins by human prostate carcinoma cell lines. Cancer Res. 1988 Apr 1;48(7):1930–1935. [PubMed] [Google Scholar]
- Visakorpi T., Kallioniemi A. H., Syvänen A. C., Hyytinen E. R., Karhu R., Tammela T., Isola J. J., Kallioniemi O. P. Genetic changes in primary and recurrent prostate cancer by comparative genomic hybridization. Cancer Res. 1995 Jan 15;55(2):342–347. [PubMed] [Google Scholar]
- Yoshikawa H., de la Monte S., Nagai H., Wands J. R., Matsubara K., Fujiyama A. Chromosomal assignment of human genomic NotI restriction fragments in a two-dimensional electrophoresis profile. Genomics. 1996 Jan 1;31(1):28–35. doi: 10.1006/geno.1996.0005. [DOI] [PubMed] [Google Scholar]
- van de Vijver M., van de Bersselaar R., Devilee P., Cornelisse C., Peterse J., Nusse R. Amplification of the neu (c-erbB-2) oncogene in human mammmary tumors is relatively frequent and is often accompanied by amplification of the linked c-erbA oncogene. Mol Cell Biol. 1987 May;7(5):2019–2023. doi: 10.1128/mcb.7.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]