Abstract
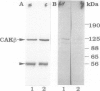
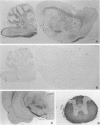
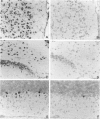
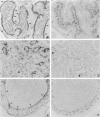
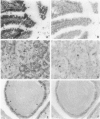
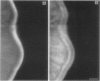
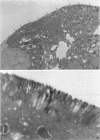
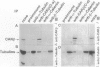
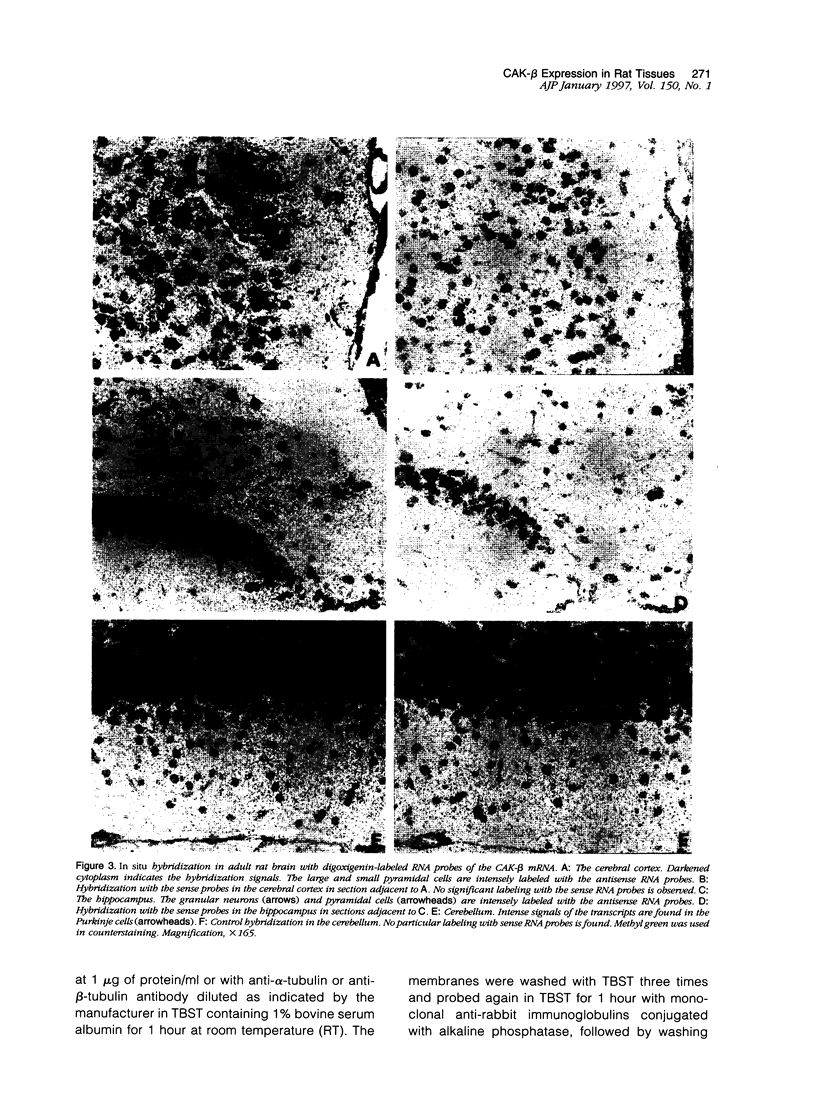
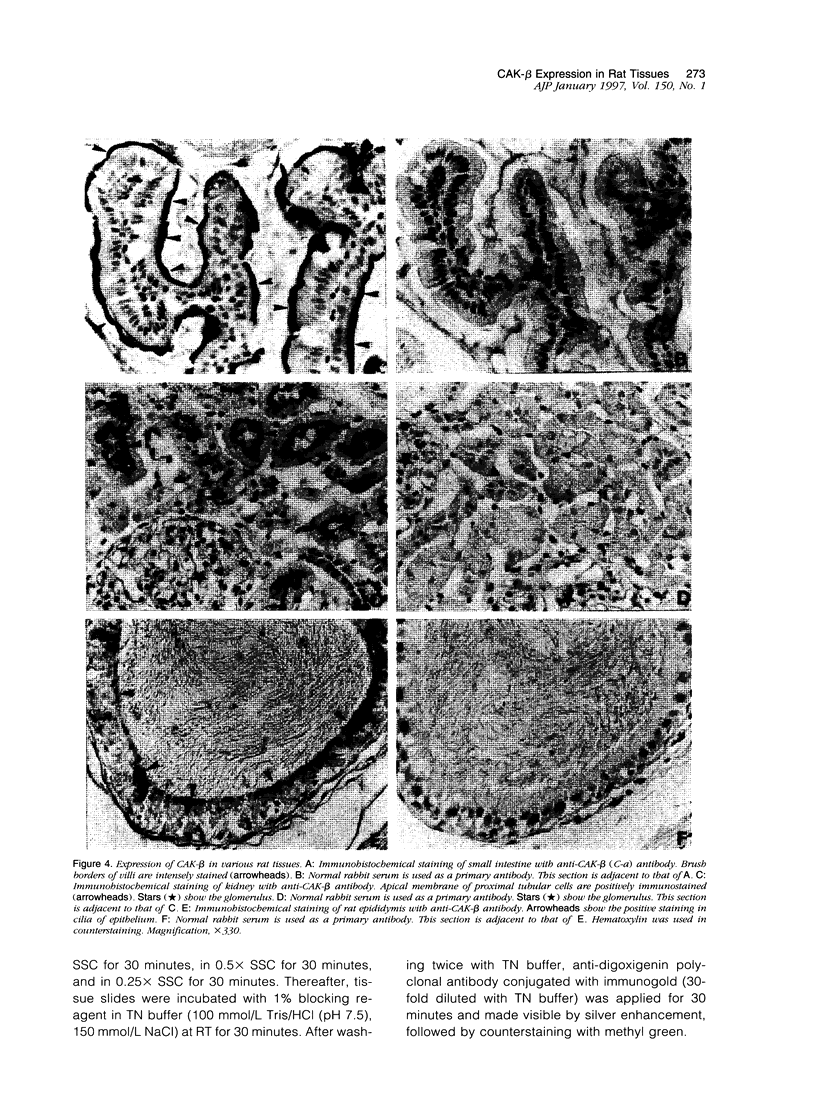
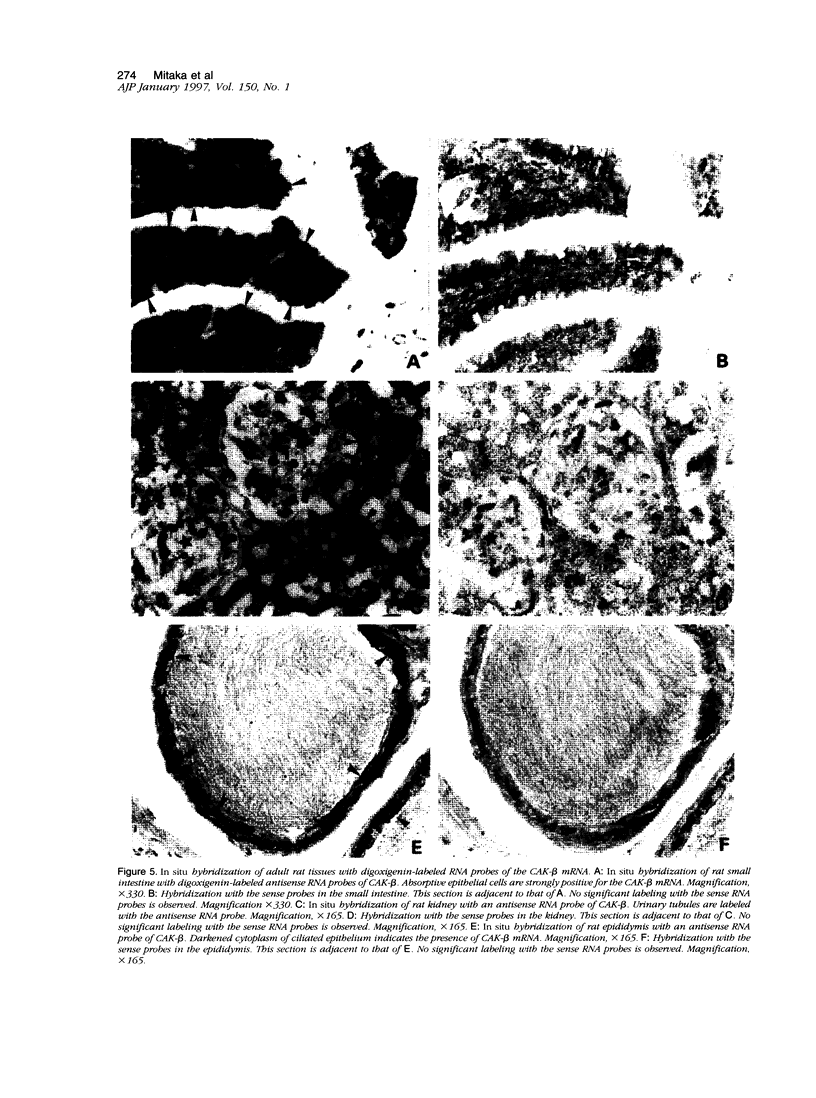
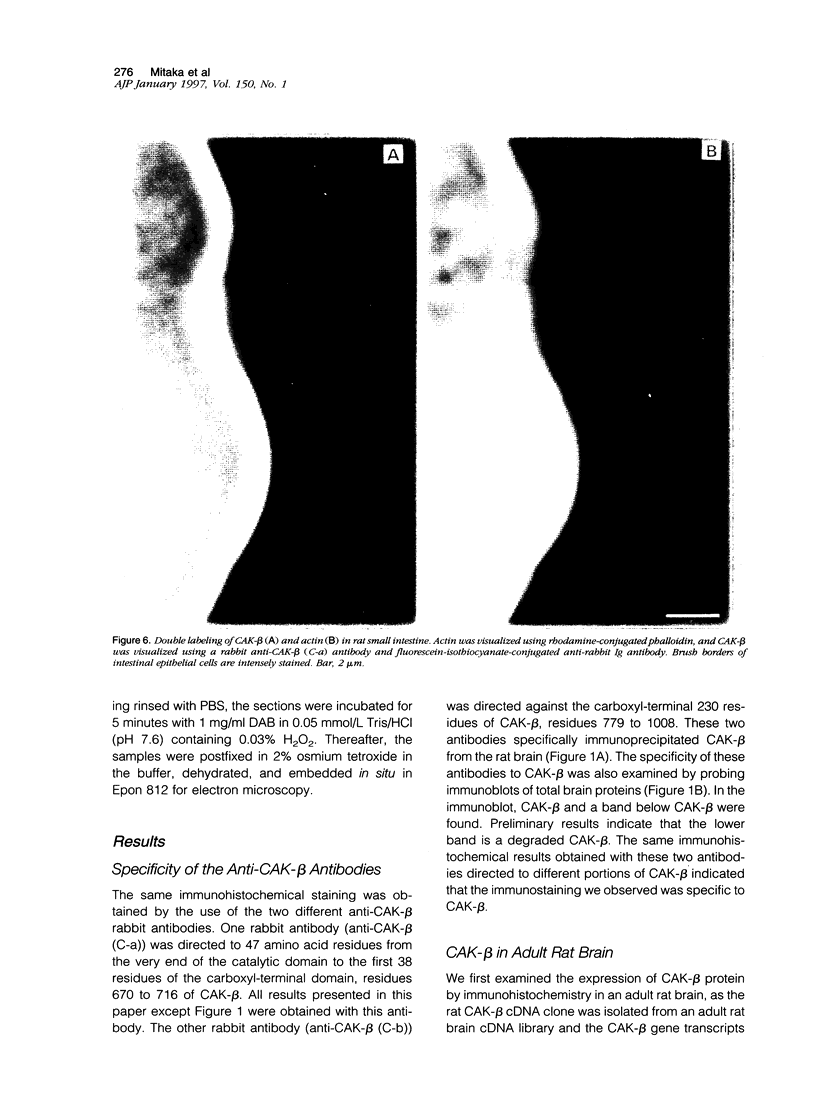
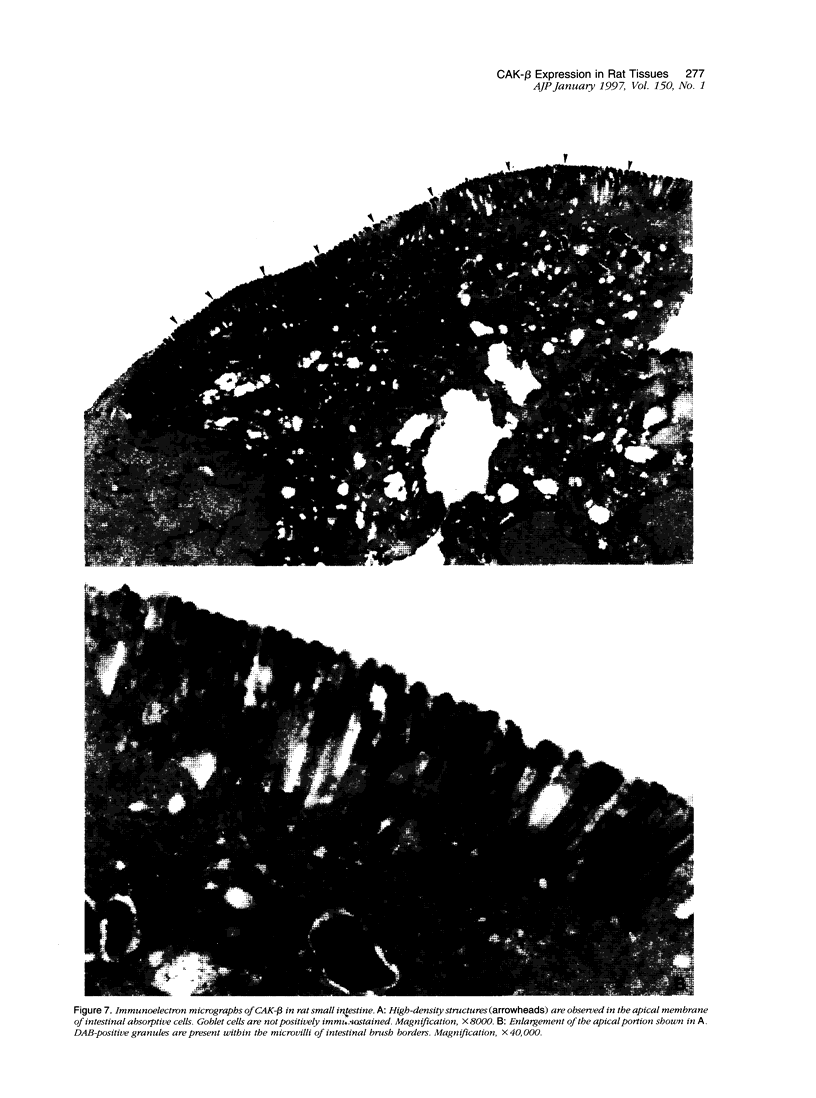
Cell adhesion kinase-beta (CAK-beta) is a protein tyrosine kinase of the focal adhesion kinase subfamily, which contains large amino- and carboxyl-terminal domains. We studied the tissue distribution of CAK-beta and its mRNA by immunohistochemical staining and in situ hybridization. In rat brain, CAK-beta was mainly found in the medulla whereas CAK-beta mRNA was expressed in most neurons, especially pyramidal cells and Purkinje cells. In the small intestine, CAK-beta protein and mRNA were detected in the absorptive epithelial cells, and the protein was concentrated in the brush border. Double immunostaining for CAK-beta and actin showed that they co-localized in the brush border of small intestine cells. Immunoelectron micrography revealed that the anti-CAK-beta antibody localized within microvilli. In the kidney, the protein was mainly expressed in proximal tubular cells, which have well developed microvilli, although CAK-beta mRNA was observed in most urinary tubular cells. In other tissues, the ciliated cells of the epididymis strongly expressed CAK-beta mRNA and CAK-beta localized in the cilia. In addition, alpha- and beta-tubulin were identified in the rat brain lysates immunoprecipitated with anti-CAK-beta antibody. The present results demonstrate that CAK-beta is present at relatively high levels in cilia, axons, and microvilli. This suggests that CAK-beta may play important roles in the functions of these structures or that the CAK-beta-related signaling pathway is closely associated with cytoskeletal components.
Full text
PDF
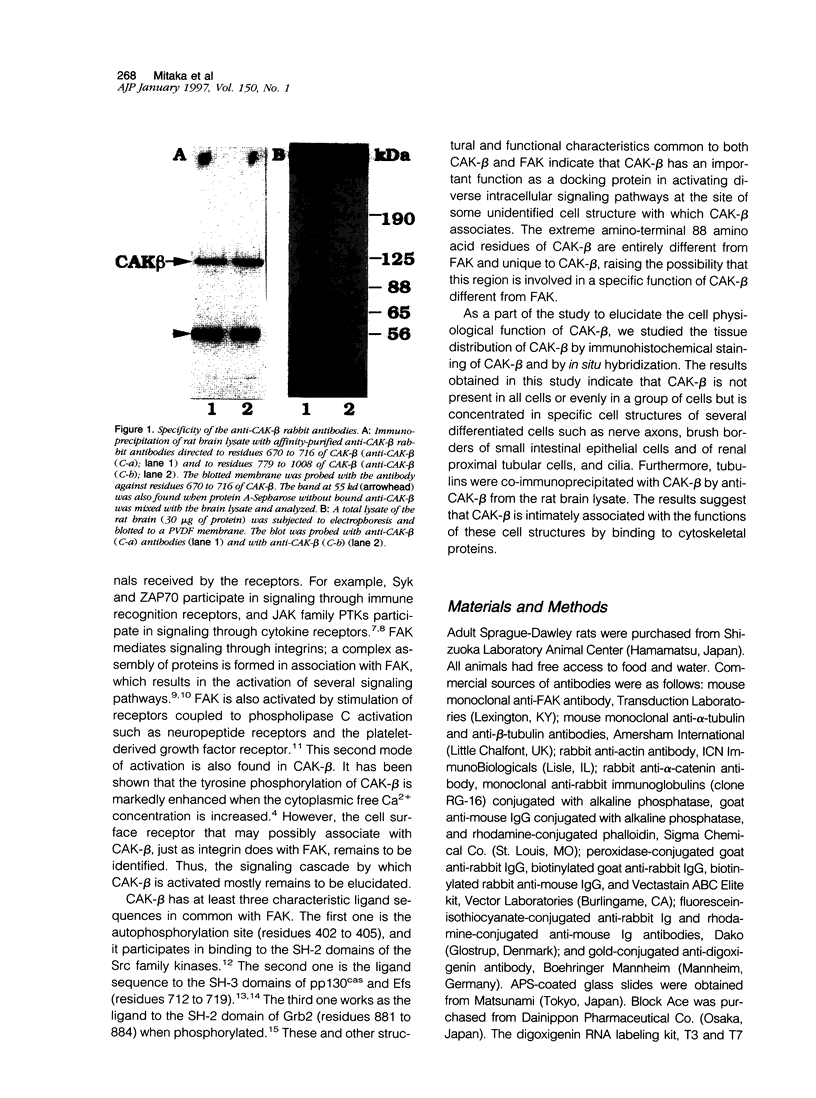






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avraham S., London R., Fu Y., Ota S., Hiregowdara D., Li J., Jiang S., Pasztor L. M., White R. A., Groopman J. E. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995 Nov 17;270(46):27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S., Greenberg M. E. Ca(2+)-dependent routes to Ras: mechanisms for neuronal survival, differentiation, and plasticity? Neuron. 1996 Feb;16(2):233–236. doi: 10.1016/s0896-6273(00)80040-9. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Karl K. A., Kiebler M. A., Kandel E. R. Focal adhesion kinase in the brain: novel subcellular localization and specific regulation by Fyn tyrosine kinase in mutant mice. Genes Dev. 1995 Aug 1;9(15):1909–1921. doi: 10.1101/gad.9.15.1909. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Herzog H., Nicholl J., Hort Y. J., Sutherland G. R., Shine J. Molecular cloning and assignment of FAK2, a novel human focal adhesion kinase, to 8p11.2-p22 by nonisotopic in situ hybridization. Genomics. 1996 Mar 15;32(3):484–486. doi: 10.1006/geno.1996.0149. [DOI] [PubMed] [Google Scholar]
- Ishino M., Ohba T., Sasaki H., Sasaki T. Molecular cloning of a cDNA encoding a phosphoprotein, Efs, which contains a Src homology 3 domain and associates with Fyn. Oncogene. 1995 Dec 7;11(11):2331–2338. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto H., Coso O. A., Gutkind J. S., Burbelo P. D., Akiyama S. K., Yamada K. M. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995 Nov;131(3):791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte T. R., Hanks S. K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Nagura K., Ishino M., Tobioka H., Kotani K., Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995 Sep 8;270(36):21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Hildebrand J. D., Shannon J. D., Fox J. W., Vines R. R., Parsons J. T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994 Mar;14(3):1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Parsons J. T. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994 Oct;6(5):705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994 Dec 22;372(6508):786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]