Abstract
Myocardial injury caused by ischemia and reperfusion comes from multiple pathogenic events, including endothelial damage, neutrophil extravasation into tissue, platelet and mast cell activation, and peroxidation of cell membrane lipids, which are followed by myocardial cell alterations resulting eventually in cell necrosis. The current study was designed to test the possible cardioprotective effect of the hormone relaxin, which has been found to cause coronary vessel dilation and to inhibit platelet and mast cell activation. Ischemia (for 30 minutes) was induced in rat hearts in vivo by ligature of the left anterior descending coronary artery; reperfusion (for 60 minutes or less if the rats died before this predetermined time) was induced by removal of the ligature. Relaxin (100 ng) was given intravenously 30 minutes before ischemia. The results obtained showed that relaxin strongly reduces 1) the extension of the myocardial areas affected by ischemia-reperfusion-induced damage, 2) ventricular arrhythmias, 3) mortality, 4) myocardial neutrophil number, 5) myeloperoxidase activity, a marker of neutrophil accumulation, 6) production of malonyldialdehyde, an end product of lipid peroxidation, 7) mast cell granule release, 8) calcium overload, and 9) morphological signs of myocardial cell injury. This study shows that relaxin can be regarded as an agent with a marked cardioprotective action against ischemia-reperfusion-induced myocardial injury.
Full text
PDF



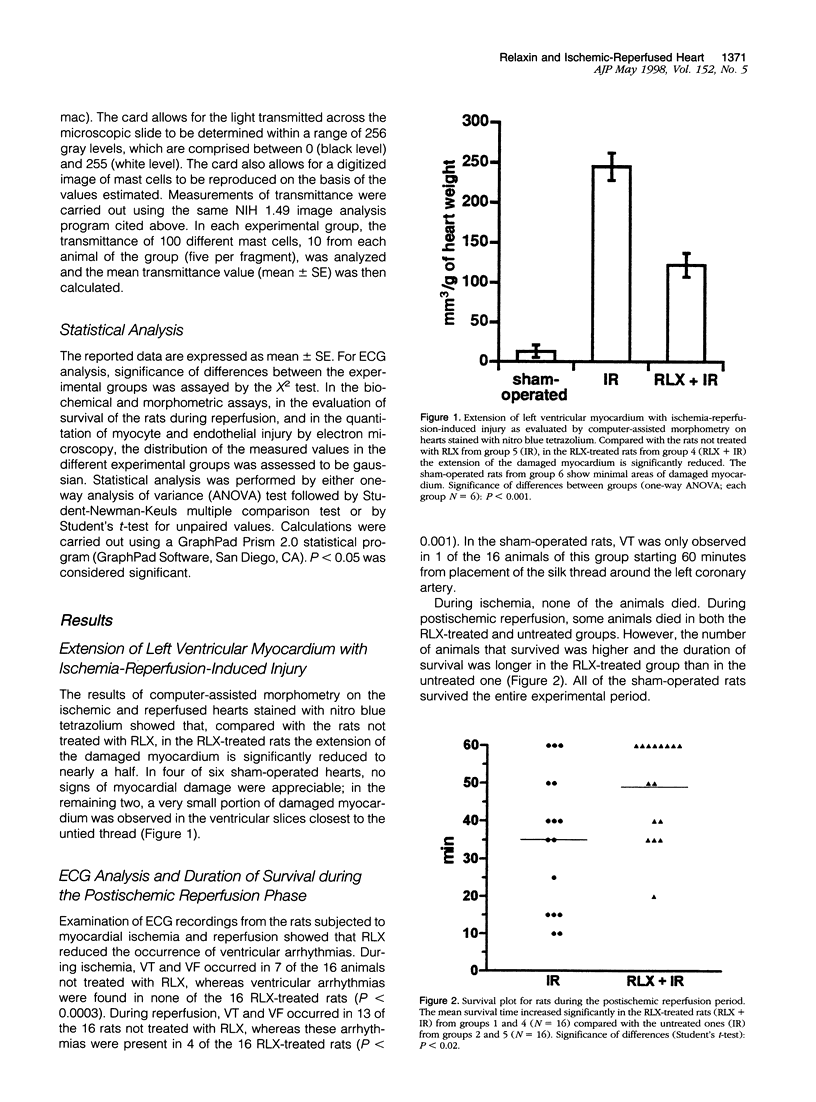
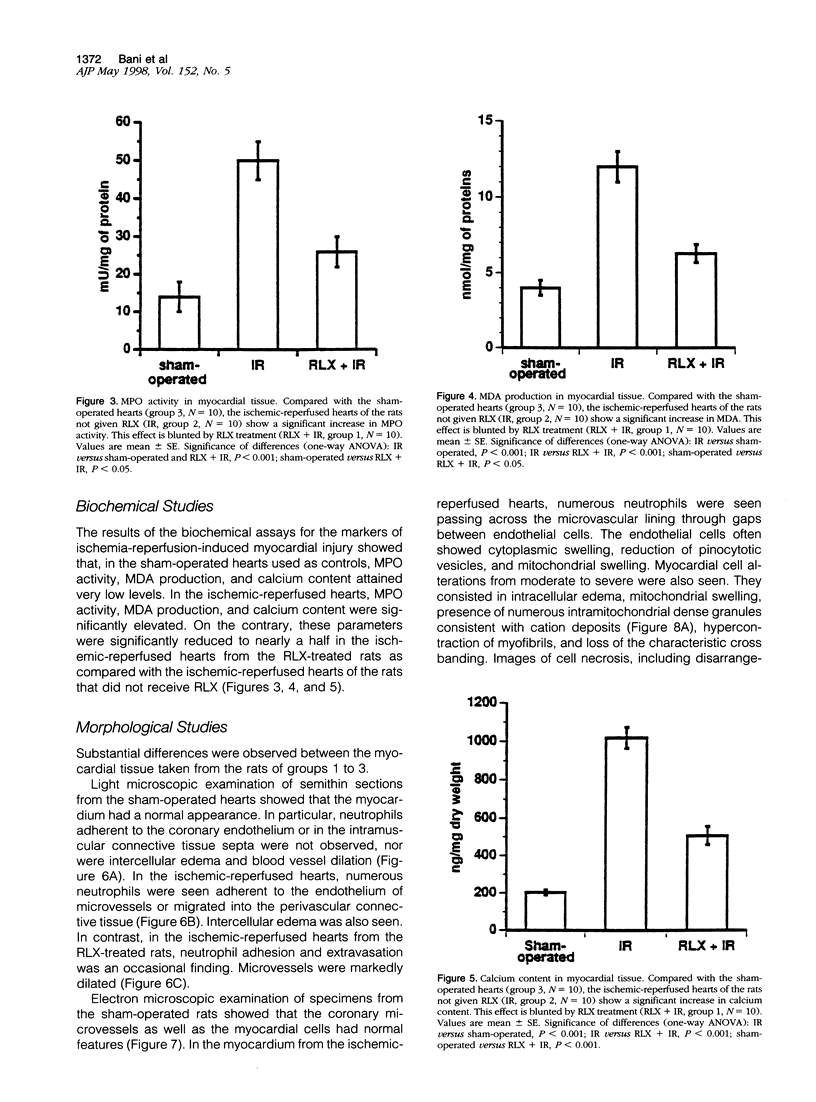


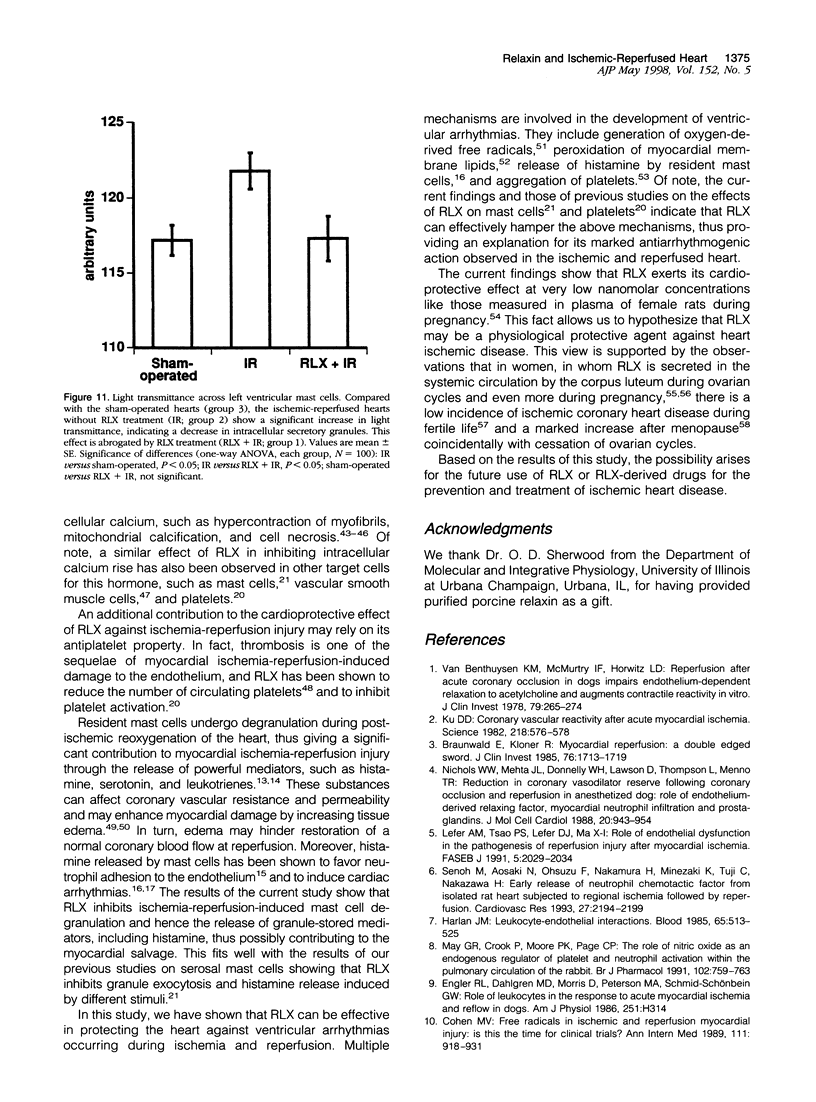

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Laughton M. J., Quinlan G. J., Gutteridge J. M. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochem J. 1989 Mar 1;258(2):617–620. doi: 10.1042/bj2580617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M., Onda M., Benedict J. B., Millard R. W. Prevention of calcium paradox-related myocardial cell injury with diltiazem, a calcium channel blocking agent. Am J Cardiol. 1982 May;49(7):1675–1681. doi: 10.1016/0002-9149(82)90245-4. [DOI] [PubMed] [Google Scholar]
- Austen K. F. The heterogeneity of mast cell populations and products. Hosp Pract (Off Ed) 1984 Sep;19(9):135-9, 143-6. doi: 10.1080/21548331.1984.11702910. [DOI] [PubMed] [Google Scholar]
- Bani-Sacchi T., Bigazzi M., Bani D., Mannaioni P. F., Masini E. Relaxin-induced increased coronary flow through stimulation of nitric oxide production. Br J Pharmacol. 1995 Sep;116(1):1589–1594. doi: 10.1111/j.1476-5381.1995.tb16377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani D., Bigazzi M., Masini E., Bani G., Sacchi T. B. Relaxin depresses platelet aggregation: in vitro studies on isolated human and rabbit platelets. Lab Invest. 1995 Nov;73(5):709–716. [PubMed] [Google Scholar]
- Bani D. Relaxin: a pleiotropic hormone. Gen Pharmacol. 1997 Jan;28(1):13–22. doi: 10.1016/s0306-3623(96)00171-1. [DOI] [PubMed] [Google Scholar]
- Bell R. J., Eddie L. W., Lester A. R., Wood E. C., Johnston P. D., Niall H. D. Relaxin in human pregnancy serum measured with an homologous radioimmunoassay. Obstet Gynecol. 1987 Apr;69(4):585–589. [PubMed] [Google Scholar]
- Bianchi S., Mugnai L. Mast cell fixation and staining in image analysis. Eur J Basic Appl Histochem. 1991;35(2):161–174. [PubMed] [Google Scholar]
- Bourdillon P. D., Poole-Wilson P. A. Effects of ischaemia and reperfusion on calcium exchange and mechanical function in isolated rabbit myocardium. Cardiovasc Res. 1981 Mar;15(3):121–130. doi: 10.1093/cvr/15.3.121. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. V. Free radicals in ischemic and reperfusion myocardial injury: is this the time for clinical trials? Ann Intern Med. 1989 Dec 1;111(11):918–931. doi: 10.7326/0003-4819-111-11-918. [DOI] [PubMed] [Google Scholar]
- Crawford M. H., Grover F. L., Kolb W. P., McMahan C. A., O'Rourke R. A., McManus L. M., Pinckard R. N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988 Dec;78(6):1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Dieterich H. A., Löffelholz K. Effect of coronary perfusion rate on the hydrolysis of exogenous and endogenous acetylcholine in the isolated heart. Naunyn Schmiedebergs Arch Pharmacol. 1977 Jan;296(2):143–148. doi: 10.1007/BF00508466. [DOI] [PubMed] [Google Scholar]
- Duncan E., Onodera T., Ashraf M. Production of hydroxyl radicals and their disassociation from myocardial cell injury during calcium paradox. Free Radic Biol Med. 1992;12(1):11–18. doi: 10.1016/0891-5849(92)90053-j. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Peter T., Hamamoto H., Mandel W. J. Electrophysiologic observations on ventricular tachyarrhythmias following reperfusion. Am Heart J. 1983 Feb;105(2):201–209. doi: 10.1016/0002-8703(83)90514-8. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Nayler W. G. Contracture and the calcium paradox. J Mol Cell Cardiol. 1985 Aug;17(8):733–745. doi: 10.1016/s0022-2828(85)80035-3. [DOI] [PubMed] [Google Scholar]
- Gelvan D., Saltman P., Powell S. R. Cardiac reperfusion damage prevented by a nitroxide free radical. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4680–4684. doi: 10.1073/pnas.88.11.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold D. E., Hillegass L., Hill D. E., Sherief H. T., Kopia G. A. Evaluation of the effect of evan's blue and triphenyltetrazolium chloride dyes on myeloperoxidase activity in canine cardiac tissue. J Pharmacol Methods. 1989 Mar;21(1):13–19. doi: 10.1016/0160-5402(89)90018-1. [DOI] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Herbaczyńska-Cedro K., Gordon-Majszak W. Attenuation by prostacyclin of adrenaline-stimulated lipid peroxidation in the myocardium. Pharmacol Res Commun. 1986 Apr;18(4):321–332. doi: 10.1016/0031-6989(86)90085-8. [DOI] [PubMed] [Google Scholar]
- Keller A. M., Clancy R. M., Barr M. L., Marboe C. C., Cannon P. J. Acute reoxygenation injury in the isolated rat heart: role of resident cardiac mast cells. Circ Res. 1988 Dec;63(6):1044–1052. doi: 10.1161/01.res.63.6.1044. [DOI] [PubMed] [Google Scholar]
- Kolodgie F. D., Virmani R., Farb A. Limitation of no reflow injury by blood-free reperfusion with oxygenated perfluorochemical (Fluosol-DA 20%). J Am Coll Cardiol. 1991 Jul;18(1):215–223. doi: 10.1016/s0735-1097(10)80242-6. [DOI] [PubMed] [Google Scholar]
- Kowey P. R., Verrier R. L., Lown B., Handin R. I. Influence of intracoronary platelet aggregation on ventricular electrical properties during partial coronary artery stenosis. Am J Cardiol. 1983 Feb;51(3):596–602. doi: 10.1016/s0002-9149(83)80103-9. [DOI] [PubMed] [Google Scholar]
- Ku D. D. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982 Nov 5;218(4572):576–578. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Lefer D. J., Ma X. L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991 Apr;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Manson J. E., Tosteson H., Ridker P. M., Satterfield S., Hebert P., O'Connor G. T., Buring J. E., Hennekens C. H. The primary prevention of myocardial infarction. N Engl J Med. 1992 May 21;326(21):1406–1416. doi: 10.1056/NEJM199205213262107. [DOI] [PubMed] [Google Scholar]
- Masini E., Bani D., Bigazzi M., Mannaioni P. F., Bani-Sacchi T. Effects of relaxin on mast cells. In vitro and in vivo studies in rats and guinea pigs. J Clin Invest. 1994 Nov;94(5):1974–1980. doi: 10.1172/JCI117549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini E., Bianchi S., Gambassi F., Palmerani B., Pistelli A., Carlomagno L., Mannaioni P. F. Ischemia reperfusion injury and histamine release in isolated and perfused guinea-pig heart: pharmacological interventions. Agents Actions. 1990 Apr;30(1-2):198–201. doi: 10.1007/BF01969037. [DOI] [PubMed] [Google Scholar]
- Masini E., Gambassi F., Giannella E., Palmerani B., Pistelli A., Carlomagno L., Mannaioni P. F. Ischemia-reperfusion injury and histamine release in isolated guinea-pig heart: the role of free radicals. Agents Actions. 1989 Apr;27(1-2):154–157. doi: 10.1007/BF02222225. [DOI] [PubMed] [Google Scholar]
- May G. R., Crook P., Moore P. K., Page C. P. The role of nitric oxide as an endogenous regulator of platelet and neutrophil activation within the pulmonary circulation of the rabbit. Br J Pharmacol. 1991 Mar;102(3):759–763. doi: 10.1111/j.1476-5381.1991.tb12246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Mehta J. L., Donnelly W. H., Lawson D., Thompson L., ter Riet M. Reduction in coronary vasodilator reserve following coronary occlusion and reperfusion in anesthetized dog: role of endothelium-derived relaxing factor, myocardial neutrophil infiltration and prostaglandins. J Mol Cell Cardiol. 1988 Oct;20(10):943–954. doi: 10.1016/s0022-2828(88)80148-2. [DOI] [PubMed] [Google Scholar]
- Ovize M., de Lorgeril M., Cathignol D., Delaye J., Renaud S. Inhibition of coronary artery thrombosis by SIN-1, a donor of nitric oxide. J Cardiovasc Pharmacol. 1990 Oct;16(4):641–645. doi: 10.1097/00005344-199010000-00017. [DOI] [PubMed] [Google Scholar]
- Parratt J. Endogenous myocardial protective (antiarrhythmic) substances. Cardiovasc Res. 1993 May;27(5):693–702. doi: 10.1093/cvr/27.5.693. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Cohen M. V., Mueller H. S. Production of free radicals and lipid peroxides in early experimental myocardial ischemia. J Mol Cell Cardiol. 1983 Oct;15(10):713–716. doi: 10.1016/0022-2828(83)90260-2. [DOI] [PubMed] [Google Scholar]
- Reynolds J. M., McDonagh P. F. Early in reperfusion, leukocytes alter perfused coronary capillarity and vascular resistance. Am J Physiol. 1989 Apr;256(4 Pt 2):H982–H989. doi: 10.1152/ajpheart.1989.256.4.H982. [DOI] [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F. Structure and function of the chemical mediators of mast cells. Prog Allergy. 1984;34:271–321. [PubMed] [Google Scholar]
- Senoh M., Aosaki N., Ohsuzu F., Nakamura H., Minezaki K., Tuji C., Nakazawa H. Early release of neutrophil chemotactic factor from isolated rat heart subjected to regional ischaemia followed by reperfusion. Cardiovasc Res. 1993 Dec;27(12):2194–2199. doi: 10.1093/cvr/27.12.2194. [DOI] [PubMed] [Google Scholar]
- Sherwood C. D., O'Byrne E. M. Purification and characterization of porcine relaxin. Arch Biochem Biophys. 1974 Jan;160(1):185–196. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- Sherwood O. D., Crnekovic V. E., Gordon W. L., Rutherford J. E. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980 Sep;107(3):691–698. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- Smith E. F., 3rd, Egan J. W., Bugelski P. J., Hillegass L. M., Hill D. E., Griswold D. E. Temporal relation between neutrophil accumulation and myocardial reperfusion injury. Am J Physiol. 1988 Nov;255(5 Pt 2):H1060–H1068. doi: 10.1152/ajpheart.1988.255.5.H1060. [DOI] [PubMed] [Google Scholar]
- Stewart D. R., Celniker A. C., Taylor C. A., Jr, Cragun J. R., Overstreet J. W., Lasley B. L. Relaxin in the peri-implantation period. J Clin Endocrinol Metab. 1990 Jun;70(6):1771–1773. doi: 10.1210/jcem-70-6-1771. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Steinhagen-Thiessen E., Schrör K. Inhibition of oxygen-centered free radical formation by the stable prostacyclin-mimetic iloprost (ZK 36 374) in acute myocardial ischemia. J Cardiovasc Pharmacol. 1984 Mar-Apr;6(2):365–366. [PubMed] [Google Scholar]
- Trudeau D. L., Freier E. F. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin Chem. 1967 Feb;13(2):101–114. [PubMed] [Google Scholar]
- VanBenthuysen K. M., McMurtry I. F., Horwitz L. D. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest. 1987 Jan;79(1):265–274. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G., O'Byrne M., Hochman J. A., Goldsmith L. T., Rifkin I., Steinetz B. G. Secretion of progesterone and relaxin by the humancorpus luteum at midpregnancy and at term. Obstet Gynecol. 1977 Dec;50(6):679–681. [PubMed] [Google Scholar]
- Wolff A. A., Levi R. Histamine and cardiac arrhythmias. Circ Res. 1986 Jan;58(1):1–16. doi: 10.1161/01.res.58.1.1. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]