Abstract
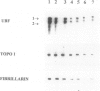
We have have studied the relationship between nucleolar function and size and cell doubling time in cancer cells. Seven human cancer cell lines characterized by different proliferation rates were used. Nucleolar functional activity was evaluated by measuring RNA polymerase I activity and expression of RNA polymerase I upstream binding factor (UBF), DNA topoisomerase I, and fibrillarin, three proteins involved in synthesis and processing of rRNA. Transcriptional activity of RNA polymerase I was strictly related to cell doubling time (r = -0.97; P < 0.001). The quantitative distribution of UBF, DNA topoisomerase I, and fibrillarin was evaluated on Western blots using specific monoclonal antibodies by densitometric analysis of autoradiographic signals. It was found to be directly related to RNA polymerase I transcriptional activity (r = 0.89, P = 0.008 for UBF; r = 0.95, P = 0.001 for DNA topoisomerase I; and r = 0.91, P = 0.004 for fibrillarin) and inversely related to cell doubling time (r = -0.87, P = 0.011 for UBF; r = -0.97, P < 0.001 for DNA topoisomerase I; and r = -0.91, P = 0.005 for fibrillarin). The nucleolar areas were measured by automated image analysis on toluidine blue-stained cells. The values of the stained nucleolar structures per cell were directly related to RNA polymerase I transcriptional activity (r = 0.94, P = 0.001) and inversely related to cell doubling time (r = -0.98, P < 0.001). The same area values of the nucleolar structures stained by toluidine blue were also closely related to the amount of UBF (r = 0.92, P = 0.003), DNA topoisomerase I (r = 0.98, P < 0.001), and fibrillarin (r = 0.95, P = 0.001), and to the in situ quantitative distribution of AgNOR proteins (r = 0.98, P < 0.001). Our results demonstrated that in cancer cells rRNA transcriptional activity and nucleolar size are inversely related to cell doubling time. Quantitative distribution of nucleolar structures within the cell represents a cytohistological parameter of the rapidity of cell proliferation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baak J. P. The relative prognostic significance of nucleolar morphometry in invasive ductal breast cancer. Histopathology. 1985 Apr;9(4):437–444. doi: 10.1111/j.1365-2559.1985.tb02826.x. [DOI] [PubMed] [Google Scholar]
- Bennion P. J., Horobin R. W., Murgatroyd L. B. The use of a basic dye (azure A or toluidine blue) plus a cationic surfactant for selective staining of RNA: a technical and mechanistic study. Stain Technol. 1975 Sep;50(5):307–313. doi: 10.3109/10520297509117080. [DOI] [PubMed] [Google Scholar]
- Bharti A. K., Olson M. O., Kufe D. W., Rubin E. H. Identification of a nucleolin binding site in human topoisomerase I. J Biol Chem. 1996 Jan 26;271(4):1993–1997. doi: 10.1074/jbc.271.4.1993. [DOI] [PubMed] [Google Scholar]
- Buckwalter C. A., Lin A. H., Tanizawa A., Pommier Y. G., Cheng Y. C., Kaufmann S. H. RNA synthesis inhibitors alter the subnuclear distribution of DNA topoisomerase I. Cancer Res. 1996 Apr 1;56(7):1674–1681. [PubMed] [Google Scholar]
- Chan E. K., Imai H., Hamel J. C., Tan E. M. Human autoantibody to RNA polymerase I transcription factor hUBF. Molecular identity of nucleolus organizer region autoantigen NOR-90 and ribosomal RNA transcription upstream binding factor. J Exp Med. 1991 Nov 1;174(5):1239–1244. doi: 10.1084/jem.174.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derenzini M., Farabegoli F., Trerè D. Relationship between interphase AgNOR distribution and nucleolar size in cancer cells. Histochem J. 1992 Dec;24(12):951–956. doi: 10.1007/BF01046500. [DOI] [PubMed] [Google Scholar]
- Derenzini M., Pession A., Trerè D. Quantity of nucleolar silver-stained proteins is related to proliferating activity in cancer cells. Lab Invest. 1990 Jul;63(1):137–140. [PubMed] [Google Scholar]
- Derenzini M., Sirri V., Pession A., Trerè D., Roussel P., Ochs R. L., Hernandez-Verdun D. Quantitative changes of the two major AgNOR proteins, nucleolin and protein B23, related to stimulation of rDNA transcription. Exp Cell Res. 1995 Jul;219(1):276–282. doi: 10.1006/excr.1995.1228. [DOI] [PubMed] [Google Scholar]
- Gamel J. W., McLean I. W. Computerized histopathologic assessment of malignant potential. II. A practical method for predicting survival following enucleation for uveal melanoma. Cancer. 1983 Sep 15;52(6):1032–1038. doi: 10.1002/1097-0142(19830915)52:6<1032::aid-cncr2820520618>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Glibetic M., Taylor L., Larson D., Hannan R., Sells B., Rothblum L. The RNA polymerase I transcription factor UBF is the product of a primary response gene. J Biol Chem. 1995 Mar 3;270(9):4209–4212. doi: 10.1074/jbc.270.9.4209. [DOI] [PubMed] [Google Scholar]
- Helpap B. Nucleolar grading of breast cancer. Comparative studies on frequency and localization of nucleoli and histology, stage, hormonal receptor status and lectin histochemistry. Virchows Arch A Pathol Anat Histopathol. 1989;415(6):501–508. doi: 10.1007/BF00718643. [DOI] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson D. E., Zahradka P., Sells B. H. Control points in eucaryotic ribosome biogenesis. Biochem Cell Biol. 1991 Jan;69(1):5–22. doi: 10.1139/o91-002. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Liu L. F., Miller K. G. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T., Stefanovsky V. Y. Promotion and regulation of ribosomal transcription in eukaryotes by RNA polymerase I. Prog Nucleic Acid Res Mol Biol. 1995;50:25–66. doi: 10.1016/s0079-6603(08)60810-7. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Pfund W. P., Mehta V. B., Trask D. K. Eukaryotic type I topoisomerase is enriched in the nucleolus and catalytically active on ribosomal DNA. EMBO J. 1985 May;4(5):1237–1243. doi: 10.1002/j.1460-2075.1985.tb03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méhes G., Pajor L. Nucleolin and fibrillarin expression in stimulated lymphocytes and differentiating HL-60 cells. A flow cytometric assay. Cell Prolif. 1995 Jun;28(6):329–336. doi: 10.1111/j.1365-2184.1995.tb00074.x. [DOI] [PubMed] [Google Scholar]
- O'Mahony D. J., Rothblum L. I. Identification of two forms of the RNA polymerase I transcription factor UBF. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3180–3184. doi: 10.1073/pnas.88.8.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Ofner D., Bankfalvi A., Riehemann K., Bier B., Böcker W., Schmid K. W. Wet autoclave pretreatment improves the visualization of silver-stained nucleolar organizer-region-associated proteins in routinely formalin-fixed and paraffin-embedded tissues. Mod Pathol. 1994 Dec;7(9):946–950. [PubMed] [Google Scholar]
- Ofner D., Hittmair A., Marth C., Ofner C., Tötsch M., Daxenbichler G., Mikuz G., Margreiter R., Schmid K. W. Relationship between quantity of silver stained nucleolar organizer regions associated proteins (Ag-NORs) and population doubling time in ten breast cancer cell lines. Pathol Res Pract. 1992 Aug;188(6):742–746. doi: 10.1016/S0344-0338(11)80171-8. [DOI] [PubMed] [Google Scholar]
- Patterson M. K., Jr Measurement of growth and viability of cells in culture. Methods Enzymol. 1979;58:141–152. doi: 10.1016/s0076-6879(79)58132-4. [DOI] [PubMed] [Google Scholar]
- Pession A., Farabegoli F., Treré D., Novello F., Montanaro L., Sperti S., Rambelli F., Derenzini M. The Ag-NOR proteins and transcription and duplication of ribosomal genes in mammalian cell nucleoli. Chromosoma. 1991 May;100(4):242–250. doi: 10.1007/BF00344158. [DOI] [PubMed] [Google Scholar]
- Ploton D., Menager M., Jeannesson P., Himber G., Pigeon F., Adnet J. J. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986 Jan;18(1):5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- Reimer G., Raska I., Tan E. M., Scheer U. Human autoantibodies: probes for nucleolus structure and function. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54(3):131–143. doi: 10.1007/BF02899205. [DOI] [PubMed] [Google Scholar]
- Roussel P., Belenguer P., Amalric F., Hernandez-Verdun D. Nucleolin is an Ag-NOR protein; this property is determined by its amino-terminal domain independently of its phosphorylation state. Exp Cell Res. 1992 Nov;203(1):259–269. doi: 10.1016/0014-4827(92)90063-e. [DOI] [PubMed] [Google Scholar]
- Sirri V., Roussel P., Gendron M. C., Hernandez-Verdun D. Amount of the two major Ag-NOR proteins, nucleolin, and protein B23 is cell-cycle dependent. Cytometry. 1997 Jun 1;28(2):147–156. [PubMed] [Google Scholar]
- Stirpe F., Novello F. Factors affecting the activity of ribonucleic acid polymerase solubilized from rat liver nuclei. Effect on ionic strength, spermine and divalent ions with native and denatured deoxyribonucleic acid. Eur J Biochem. 1970 Sep;15(3):505–512. doi: 10.1111/j.1432-1033.1970.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Trerè D., Pession A., Derenzini M. The silver-stained proteins of interphasic nucleolar organizer regions as a parameter of cell duplication rate. Exp Cell Res. 1989 Sep;184(1):131–137. doi: 10.1016/0014-4827(89)90371-6. [DOI] [PubMed] [Google Scholar]
- Voit R., Kuhn A., Sander E. E., Grummt I. Activation of mammalian ribosomal gene transcription requires phosphorylation of the nucleolar transcription factor UBF. Nucleic Acids Res. 1995 Jul 25;23(14):2593–2599. doi: 10.1093/nar/23.14.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A. The vanishing lung. Lab Invest. 1995 Jul;73(1):1–2. [PubMed] [Google Scholar]
- Zhang H., Wang J. C., Liu L. F. Involvement of DNA topoisomerase I in transcription of human ribosomal RNA genes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1060–1064. doi: 10.1073/pnas.85.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]