Abstract
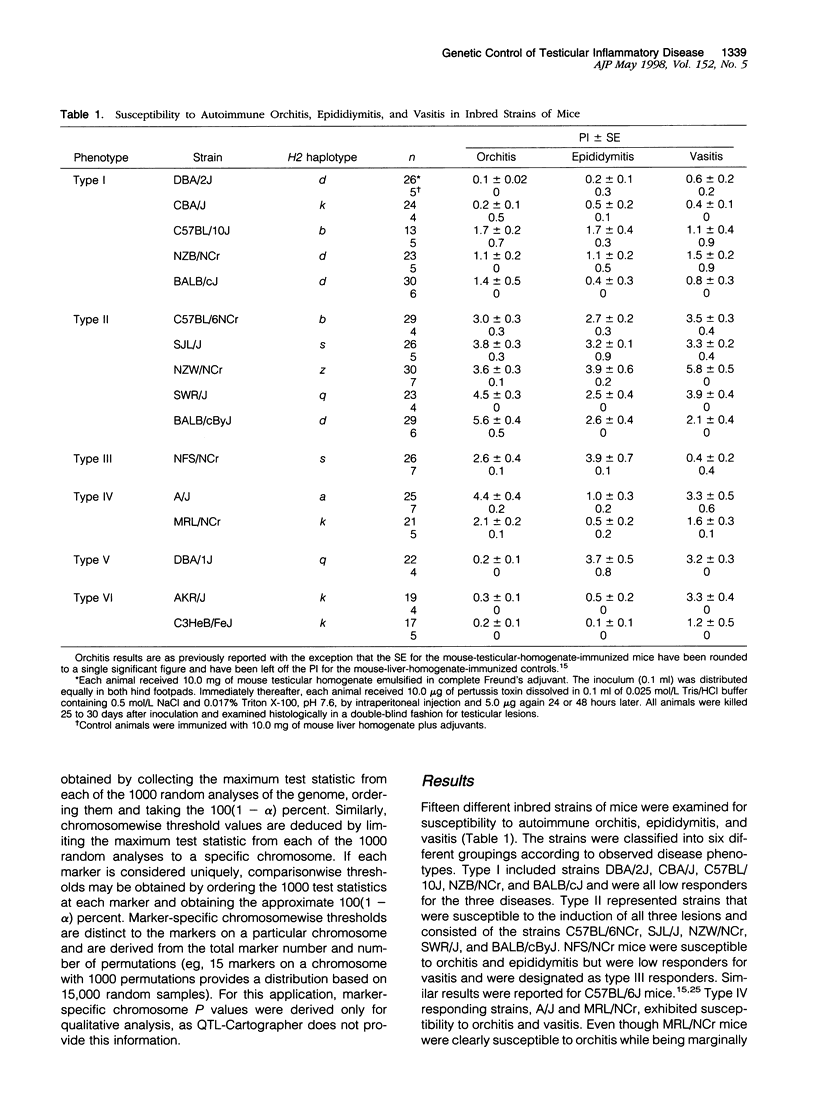
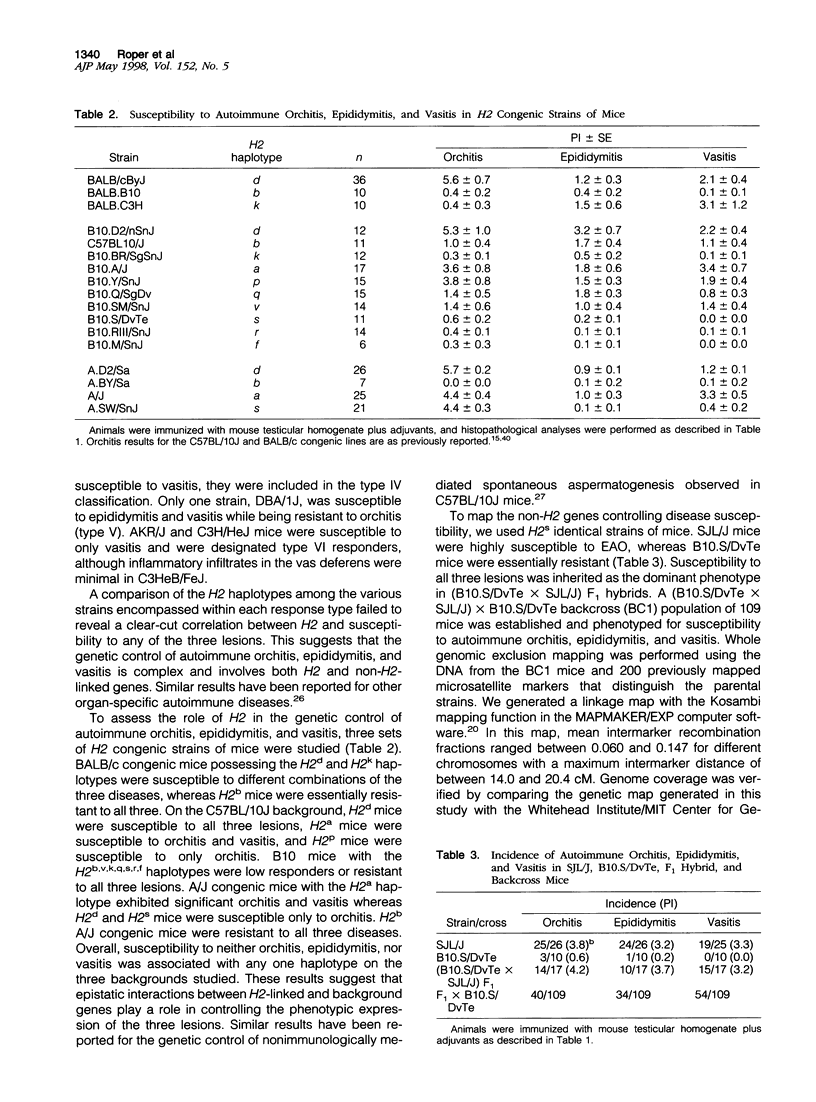
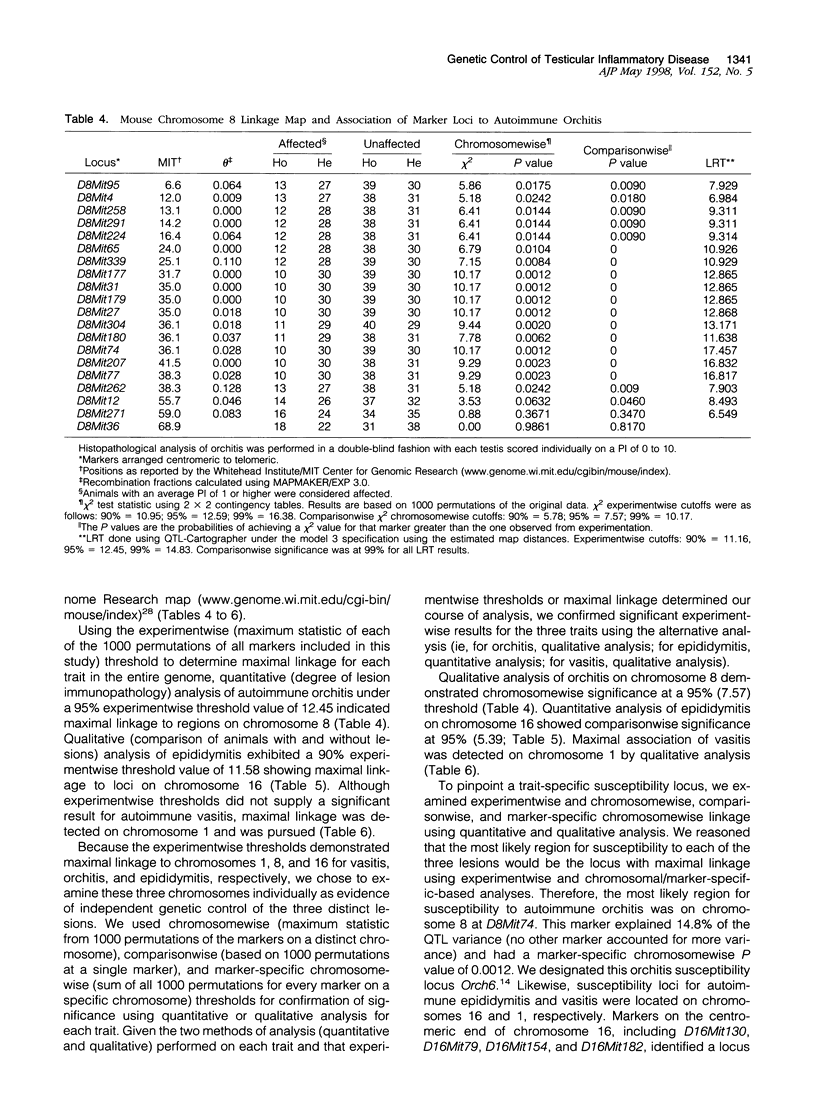
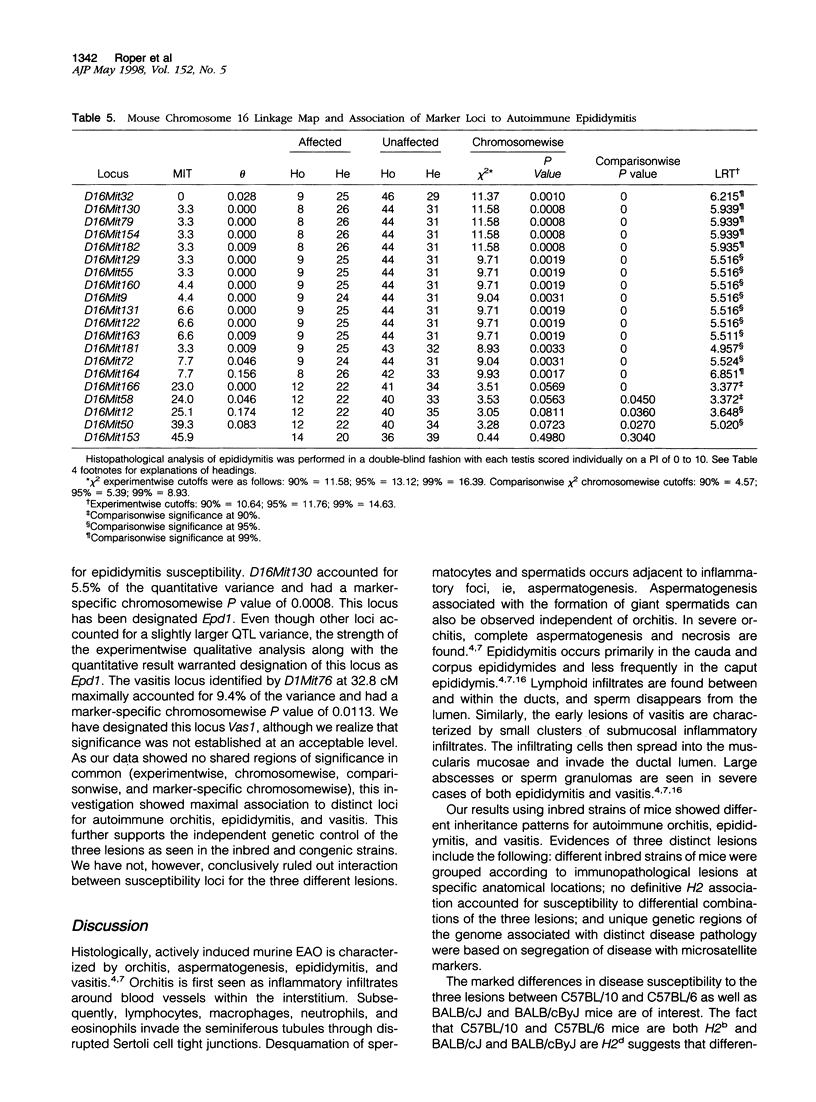
Experimental allergic orchitis (EAO), the principle animal model of noninfectious testicular inflammatory disease, is a genetically determined phenotype. Classical EAO, induced by inoculation with testicular homogenate and the appropriate adjuvants, is characterized by inflammatory infiltrates in the testis (orchitis), epididymis (epididymitis), and vas deferens (vasitis). In this study, the genetic control of susceptibility and resistance to these three lesions was analyzed in the mouse. The results obtained with independent inbred strains and H2 congenic mice show that the genetic control of all three lesions is complex and involves both H2 and non-H2-linked genes. Whole-genome exclusion mapping was performed on a backcross population segregating for all three phenotypes. Permutation-derived thresholds provided experimentwise, chromosomewise, comparisonwise, and marker-specific chromosomewise thresholds for declaration of significant regions linked to marker loci. Unique loci were identified on chromosome 8 for orchitis, chromosome 16 for epididymitis, and chromosome 1 for vasitis and have been designated as Orch6, Epd1, and Vas1, respectively. These results show that autoimmune orchitis, epididymitis, and vasitis are immunogenetically distinct lesions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenhorn E. P., Stranford S. A. Genetic factors in demyelinating diseases: genes that control demyelination due to experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalitis virus. Reg Immunol. 1992 Sep-Oct;4(5):331–343. [PubMed] [Google Scholar]
- Churchill G. A., Doerge R. W. Empirical threshold values for quantitative trait mapping. Genetics. 1994 Nov;138(3):963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W. F., Miller J. C., Steen R. G., Merchant M., Damron D., Nahf R., Gross A., Joyce D. C., Wessel M., Dredge R. D. A genetic map of the mouse with 4,006 simple sequence length polymorphisms. Nat Genet. 1994 Jun;7(2 Spec No):220–245. doi: 10.1038/ng0694supp-220. [DOI] [PubMed] [Google Scholar]
- Dietrich W. F., Miller J., Steen R., Merchant M. A., Damron-Boles D., Husain Z., Dredge R., Daly M. J., Ingalls K. A., O'Connor T. J. A comprehensive genetic map of the mouse genome. Nature. 1996 Mar 14;380(6570):149–152. doi: 10.1038/380149a0. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Hiramine C., Hojo K. A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. I. Immunological and histological studies. Clin Exp Immunol. 1991 Jan;83(1):137–142. doi: 10.1111/j.1365-2249.1991.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989 Jan;121(1):185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Green P., Abrahamson J., Barlow A., Daly M. J., Lincoln S. E., Newberg L. A., Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987 Oct;1(2):174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- McClive P. J., Huang D., Morahan G. C57BL/6 and C57BL/10 inbred mouse strains differ at multiple loci on chromosome 4. Immunogenetics. 1994;39(4):286–288. doi: 10.1007/BF00188793. [DOI] [PubMed] [Google Scholar]
- Meeker N. D., Hickey W. F., Korngold R., Hansen W. K., Sudweeks J. D., Wardell B. B., Griffith J. S., Teuscher C. Multiple loci govern the bone marrow-derived immunoregulatory mechanism controlling dominant resistance to autoimmune orchitis. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5684–5688. doi: 10.1073/pnas.92.12.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person P. L., Snoek M., Demant P., Woodward S. R., Teuscher C. The immunogenetics of susceptibility and resistance to murine experimental allergic orchitis. Reg Immunol. 1992 Sep-Oct;4(5):284–297. [PubMed] [Google Scholar]
- Robison R., Tung K. S., Meeker N. D., Monson F. G., Teuscher C. A murine model of spontaneous aspermatogenesis: linkage to H-2. J Reprod Immunol. 1994 May;26(3):251–260. doi: 10.1016/0165-0378(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y., Himeno K., Sanui H., Yoshida S., Nomoto K. Experimental allergic orchitis in mice. I. A new model induced by immunization without adjuvants. Clin Immunol Immunopathol. 1985 Dec;37(3):360–368. doi: 10.1016/0090-1229(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Sudweeks J. D., Todd J. A., Blankenhorn E. P., Wardell B. B., Woodward S. R., Meeker N. D., Estes S. S., Teuscher C. Locus controlling Bordetella pertussis-induced histamine sensitization (Bphs), an autoimmune disease-susceptibility gene, maps distal to T-cell receptor beta-chain gene on mouse chromosome 6. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3700–3704. doi: 10.1073/pnas.90.8.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C., Blankenhorn E. P., Hickey W. F. Differential susceptibility to actively induced experimental allergic encephalomyelitis and experimental allergic orchitis among BALB/c substrains. Cell Immunol. 1987 Dec;110(2):294–304. doi: 10.1016/0008-8749(87)90124-9. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Gasser D. L., Woodward S. R., Hickey W. F. Experimental allergic orchitis in mice. VI. Recombinations within the H-2S/H-2D interval define the map position of the H-2-associated locus controlling disease susceptibility. Immunogenetics. 1990;32(5):337–344. doi: 10.1007/BF00211648. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Meeker N. D., Livingstone K. D., Sudweeks J. D., Griffith J. S., Wardell B. B., Hickey W. F. Experimental allergic orchitis in mice. VII. Preliminary characterization of the aspermatogenic autoantigens responsible for eliciting actively and passively induced disease. J Reprod Immunol. 1994 May;26(3):233–249. doi: 10.1016/0165-0378(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Smith S. M., Goldberg E. H., Shearer G. M., Tung K. S. Experimental allergic orchitis in mice. I. Genetic control of susceptibility and resistance to induction of autoimmune orchitis. Immunogenetics. 1985;22(4):323–333. doi: 10.1007/BF00430916. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Smith S. M., Tung K. S. Experimental allergic orchitis in mice: III. Differential susceptibility and resistance among BALB/c sublines. J Reprod Immunol. 1987 Mar;10(3):219–230. doi: 10.1016/0165-0378(87)90088-x. [DOI] [PubMed] [Google Scholar]
- Teuscher C., Wardell B. B., Lunceford J. K., Michael S. D., Tung K. S. Aod2, the locus controlling development of atrophy in neonatal thymectomy-induced autoimmune ovarian dysgenesis, co-localizes with Il2, Fgfb, and Idd3. J Exp Med. 1996 Feb 1;183(2):631–637. doi: 10.1084/jem.183.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Aitman T. J., Cornall R. J., Ghosh S., Hall J. R., Hearne C. M., Knight A. M., Love J. M., McAleer M. A., Prins J. B. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991 Jun 13;351(6327):542–547. doi: 10.1038/351542a0. [DOI] [PubMed] [Google Scholar]
- Tung K. S., Teuscher C. Mechanisms of autoimmune disease in the testis and ovary. Hum Reprod Update. 1995 Jan;1(1):35–50. doi: 10.1093/humupd/1.1.35. [DOI] [PubMed] [Google Scholar]
- Tung K. S., Yule T. D., Mahi-Brown C. A., Listrom M. B. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol. 1987 Feb 1;138(3):752–759. [PubMed] [Google Scholar]
- Voisin G. A., Toullet F. Autoimmune aspermatogenic orchitis (A.I.A.O.). A model for three possible mechanisms of autoimmune lesions. Folia Allergol (Roma) 1971 Jul-Aug;18(4):310–316. [PubMed] [Google Scholar]
- Vyse T. J., Todd J. A. Genetic analysis of autoimmune disease. Cell. 1996 May 3;85(3):311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- Wardell B. B., Michael S. D., Tung K. S., Todd J. A., Blankenhorn E. P., McEntee K., Sudweeks J. D., Hansen W. K., Meeker N. D., Griffith J. S. Aod1, the immunoregulatory locus controlling abrogation of tolerance in neonatal thymectomy-induced autoimmune ovarian dysgenesis, maps to mouse chromosome 16. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4758–4762. doi: 10.1073/pnas.92.11.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Todd J. A., Peterson L. B. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. doi: 10.1146/annurev.iy.13.040195.001143. [DOI] [PubMed] [Google Scholar]
- Yule T. D., Tung K. S. Experimental autoimmune orchitis induced by testis and sperm antigen-specific T cell clones: an important pathogenic cytokine is tumor necrosis factor. Endocrinology. 1993 Sep;133(3):1098–1107. doi: 10.1210/endo.133.3.8103448. [DOI] [PubMed] [Google Scholar]


